Pregabalin, gabapentin are often considered first-line treatments for various neuropathic pain syndromes, generally irrespective of cause. Because the products are so variable, this article compares the pharmacokinetics (PK) and pharmacodynamics (PD) of pregabalin with various gabapentin formulations, and also covers conversion regimens.
Pregabalin, Gabapentin which one is better?
www.rxharun.com
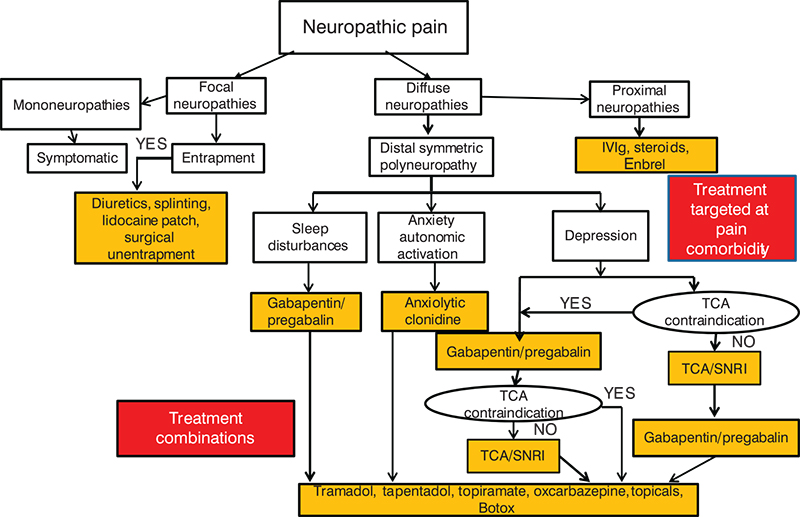
Neuropathic pain causes significant morbidity in the United States.
The incidence of peripheral neuropathy is estimated at about 2.4% of the population. Of the 14 million US individuals with diabetes, roughly 25% experience painful diabetic neuropathy.
Despite advances in vaccination for varicella zoster virus, around 25% of patients with a herpes zoster infection will develop persistent neuropathic pain. More than 85% of patients with neuropathic pain caused by peripheral neuropathy will require pharmacotherapy.
Unfortunately, there are few head-to-head trials comparing agents for neuropathic pain, so selecting the best option can be difficult.
Pharmacokinetics of pregabalin, gabapentin
Both pregabalin and gabapentin are antiepileptic medications that bare structural resemblance to gamma-aminobutyric acid (GABA), though neither agent has activity in GABA’s neuronal systems.Although the exact mechanism of action is somewhat unclear, the drugs’ efficacy in neuropathic pain is linked to their ability to bind to voltage-gated calcium channels in the central nervous system (CNS), specifically to the alpha-2-delta protein. This binding decreases neurotransmitter release in the CNS as a result of reduced calcium influx through the gated channels.
Gabapentin is indicated as adjunct therapy for partial seizures and postherpetic neuralgia.Pregabalin is indicated for the same uses as gabapentin, plus the management of fibromyalgia and neuropathic pain associated with diabetes, specifically diabetic neuropathy.
Overall, the pharmacokinetic profiles of these 2 medications are somewhat similar, but they also have some significant differences.
For example, both drugs are structurally similar to the amino acid leucine. Because of this, they can both undergo facilitated transport across cellular membranes through system L-amino acid transporters. This is the major form of absorption for gabapentin and pregabalin, with the exception of an extended-release gabapentin prodrug to be discussed later.
However, pregabalin may either have an additional system of absorption or be better transported than gabapentin, as it is almost completely absorbed, while gabapentin is not. In addition, absorption of gabapentin is limited to the small intestine, while pregabalin is absorbed throughout the small intestine and extending to the ascending colon.
Gabapentin is more slowly and variably absorbed, with peak plasma concentrations around 3 hours post-dose. Pregabalin is quickly absorbed, with the maximum rate of absorption being 3 times that of gabapentin. It reaches peak blood concentrations within an hour after ingestion.
Absorption of gabapentin is saturable, leading to a non-linear pharmacokinetic profile. As gabapentin doses increase, the area under the curve (AUC) does not follow proportionally. Unlike gabapentin, absorption of pregabalin is not saturable, and the drug has a linear pharmacokinetic profile.
The bioavailability of generic gabapentin in tablet and capsule formulations equivalent to brand-name Neurontin is about 80% at lower doses such as 100 mg every 8 hours, but only 27% bioavailable at doses of 1600 mg every 8 hours.This differs greatly from pregabalin, which boasts a greater than 90% bioavailability across a dosage range from 75 mg to 900 mg daily in divided doses.
Gabapentin’s bioavailability for its intended patient population is also more variable than pregabalin’s bioavailability. Variability among patients is believed to be 10% to 15% with pregabalin and 20% to 30% with gabapentin.
Finally, food increases the AUC of gabapentin by about 10%, with no change in time to maximum concentration (tmax). In contrast, the AUC of pregabalin is unaffected by food, though absorption is slower.
Distribution of gabapentin and pregabalin is very similar. Neither agent is bound by great extent to any plasma proteins, decreasing the likelihood of drug interactions due to protein binding. Both have high aqueous solubility, and the volume of distribution of each is similar (0.8 L/kg and 0.5 L/kg for gabapentin and pregabalin, respectively).
Drug-drug interactions are unlikely for both pregabalin and gabapentin. Neither pregabalin nor gabapentin is affected by cytochrome (CYP) drug interactions, as neither drug is metabolized by CYP enzymes. Both undergo metabolism to a negligible extent (<1%).
Renal excretion is the major method of both drugs’ elimination from the body. Agents that decrease small bowel motility can theoretically cause an increase in the absorption of gabapentin, because it is not completely absorbed. However, as pregabalin is more than 90% absorbed, its absorption is not affected by changes in small bowel motilit
Gabapentin formulations
Gabapentin is also available in 2 extended-release formulations: a tablet (Gralise) and a gastro-retentive prodrug, gabapentin enacarbil (Horizant).Both have different pharmacokinetics and are not interchangeable with standard formulations, the original of which is Neurontin. Side effects of these newer formulations are similar to standard formulations.
Gralise is indicated for postherpetic neuralgia and taken as an 1800 mg maintenance dose once a day. The AUC of 1800 mg of Gralise is slightly less than 1800 mg of the standard formulation. In addition, the average maximum concentration (Cmax) of Gralise is slightly higher than 1800 mg of the standard form, and minimum concentration (Cmin) is slightly lower. Finally, Tmax is increased in the extended-release form.
Although food does not palpably affect the AUC of standard forms of gabapentin, Gralise should be taken with food because the bioavailability is greatly increased (33% to 118%, depending on the meal’s fat content).
Horizant is a prodrug of gabapentin indicated for postherpetic neuralgia and restless leg syndrome. The recommended maintenance dose is 600 mg twice a day. Doses greater than 1200 mg daily are not recommended, as side effects increase without a corresponding increase in efficacy.
Bioavailability of gabapentin enacarbil is about 75%, which is somewhat improved over the standard formulation. It is absorbed through the small intestine through a proton-linked monocarboxylate transporter (MCT-1).
Unlike the original formulation, absorption of gabapentin enacarbil is not saturated at high doses, as MCT-1 is expressed in high levels in the intestinal tract. The drug undergoes near-complete first pass hydrolysis to gabapentin by non-specific carboxylesterases mainly in enterocytes, as well as in the liver to a lessor degree.
Consumption of alcohol increases the release of gabapentin enacarbil from the extended-release tablet. Therefore, alcohol should be avoided when taking Horizant due to increased risk of side effects.
Like standard formulations of gabapentin, Gralise and Horizant are not metabolized to an appreciable extent by phase I metabolism, and they are neither a substrate nor an inhibitor of p-glycoprotein. All forms of gabapentin must be adjusted in renal dysfunction, similar to standard formulations.
One review found that these extended-release formulations have similar efficacy to standard ones, and they might also have fewer adverse events.
Both pregabalin and gabapentin are well tolerated. Dizziness and somnolence are the most common side effects in both drugs (>20% seen in gabapentin). Confusion and peripheral edema have also been reported with gabapentin.
With both drugs, side effects are dose dependent and reversible if the medication is discontinued. The abrupt discontinuation of any form of gabapentin is not recommended because withdrawal symptoms such as anxiety, insomnia, nausea, pain, and sweating may present. When discontinuing gabapentin it is recommended to taper the dose over a week at least.
As with all antiepileptic drugs, increased risk of suicidal ideation is possible.
Pharmacodynamics of pregabalin and gabapentin
Gabapentin and pregabalin vary in terms of binding affinity and potency. Pregabalin has an increased binding affinity for the alpha-2-delta protein and is a more potent analgesic in neuropathic pain compared with gabapentin. One study developed a population pharmacokinetic model comparing pregabalin with gabapentin. The authors calculated values for the concentration of the drug that will give one-half the maximum pharmacologic response (EC50) and used these values to assess potency of the 2 medications.
Based on studies of gabapentin and pregabalin in epilepsy, the EC50 values of pregabalin and gabapentin were estimated to be about 9.77 mg/mL and 23.9 mg/mL, respectively.9 From these data, pregabalin was estimated to be about 2.4 times more potent. For neuropathic pain, pregabalin’s potency ratio may be even greater.
Using studies in postherpetic neuralgia, the EC50 values of pregabalin and gabapentin were estimated to be about 4.21 mg/mL and 11.7 mg/mL, respectively. Based on these values, pregabalin was estimated to be about 2.8 times more potent than gabapentin.
Pregabalin and gabapentin differ somewhat in terms of their dose-response curves.
One study analyzed data from phase 2 trials of gabapentin and pregabalin and created a pharmacodynamic model. The authors found that in patients with postherpetic neuralgia, mean pain scores decreased as the dose of both gabapentin and pregabalin increased.
However, the study also uncovered a plateau of gabapentin’s effect on reducing pain at around 3600 mg/day. In contrast, the pain-relieving effect of pregabalin continued to increase up to the maximum dose of 450 mg/day.
Pregabalin also exhibited a steeper dose-response curve than gabapentin. Based on the dose-response curves predicted in this model, a dose of 450 mg/day of pregabalin equates to about 3600 mg/day of gabapentin.
Converting from gabapentin to pregabalin
For clinicians who wish to convert patients from gabapentin to pregabalin, there are a few studies that reviewed such a conversion.One cohort study reviewed the utility of switching patients with neuropathic pain due to peripheral neuropathy from gabapentin to pregabalin.10 The study followed patients who were switched from gabapentin to pregabalin and then compared them to those who stayed on gabapentin. The authors also stratified the pregabalin group further into those who responded well or poorly to gabapentin, with gabapentin stopped after the nighttime dose and pregabalin started the following morning.
Dosages were switched using the following algorithm:
- Gabapentin ≤900 mg/day → pregabalin 150mg/day
- Gabapentin 901 mg/day to 1500 mg/day → pregabalin 225 mg/day
- Gabapentin 1501 mg/day 2100 mg/day → pregabalin 300 mg/day
- Gabapentin 2101 mg/day 2700 mg/day → pregabalin 450 mg/day
- Gabapentin >2700 mg/day → pregabalin 600 mg/day
This rapid change was generally well tolerated by patients.
Diabetic Peripheral Neuropathic Pain
Initial: 50 mg PO q8hr
Maintenance: May increase to 100 mg PO q8hr within 1 week, as needed; not to exceed 300 mg/day
Postherpetic Neuralgia
Initial: 150-300 mg/day PO divided q8-12hr
Maintenance: May increase to 300 mg/day divided q8-12hr after 1 week, as needed
Fibromyalgia
Initial: 150 mg/day PO divided q12hr
Maintenance: May increase to 300-450 mg/day divided q12hr after 1 week, as needed
Epilepsy
Initial: 150 mg/d divided q8-12hr PO
Maintenance: May increase to 600 mg/day PO divided q8-12hr, as needed
Neuropathic Pain With Spinal Cord Injury
Initial: 150 mg/day PO divided q12hr; may increase within 1 week to 300 mg/day PO divided q12hr
If there is insufficient pain relief after 2-3 weeks and 300 mg/day dose is tolerated, may increase dose again up to 600 mg/day PO divided q12hr
Dosing Modifications
Renal impairment (CrCl 30-60 mL/min)
- Decrease dose by 50% divided bid/tid
Renal impairment (CrCl 15-30 mL/min)
- If 150 mg/day in normal renal function: Decrease dose to 25-50 mg/day; administer qDay or divided bid
- If 300 mg/day in normal renal function: Decrease dose to 75 mg/day; administer qDay or divided bid
- If 450 mg/day in normal renal function: Decrease dose to 100-150 mg/day; administer qDay or divided bid
- If 600 mg/day in normal renal function: Decrease dose to 150 mg/day; administer qDay or divided bid
Renal impairment (CrCl <15 mL/min)
- If 150 mg/day in normal renal function: Decrease dose to 25 mg/day; single daily dose
- If 300 mg/day in normal renal function: Decrease dose to 25-50 mg/day; single daily dose
- If 450 mg/day in normal renal function: Decrease dose to 50-75 mg/day; single daily doseof divided bid
- If 600 mg/day in normal renal function: Decrease dose to 75 mg/day; single daily dose
Renal impairment (supplemental dosage following hemodialysis)
- 25 mg qDay regimen: Take 1 supplemental dose of 25 mg or 50 mg
- 25-50 mg qDay regimen: Take 1 supplemental dose of 50 mg or 75 mg
- 50-75 mg qDay regimen: Take 1 supplemental dose of 75 mg or 100 mg
- 75 mg qDay regimen: Take 1 supplemental dose of 100 mg or 150 mg
The authors found that those who responded well to gabapentin and those who did not showed additional benefit with decreased pain when they were switched to pregabalin. Patients taking pregabalin also had improved pain control compared with those who remained on gabapentin.Switching to pregabalin resulted in improved pain relief and also fewer adverse events. This was particularly true for patients who previously responded to gabapentin.
Patients who experienced adverse events with gabapentin were more likely to also experience adverse events with pregabalin. These patients were also more likely to discontinue use of pregabalin than those who responded well to both gabapentin and pregabalin.
Another small trial compared the degree of pain relief with gabapentin to pregabalin in patients with postherpetic neuralgia in order to more closely determine equivalent dosing between the 2 medications.
Patients were switched from gabapentin to pregabalin using one-sixth the dose of gabapentin with unchanged dosage frequency. After switching medications, patients reported similar pain relief and side effects, with the exception of an increased incidence of peripheral edema in the pregabalin group.
The authors concluded that the analgesic effect of pregabalin was about 6 times that of gabapentin.
Other studies have looked at methods for converting gabapentin to pregabalin. One such trial used population pharmacokinetic models to examine 2 possible scenarios for converting gabapentin to pregabalin, using a ratio of 6:1 gabapentin to pregabalin.
The first scenario involved discontinuing gabapentin and immediately starting pregabalin at the next scheduled dosing period. The other option included a gradual transition from gabapentin to pregabalin.
In this second scenario, the gabapentin dose was decreased by 50%, and 50% of the desired pregabalin dose was given concurrently for 4 days. After this time, gabapentin was discontinued and pregabalin was increased to full desired dose.
The model looked at transitioning patients from gabapentin to pregabalin at various doses, including:
- Gabapentin 900 mg/day → pregabalin 150 mg/day
- Gabapentin 1800 mg/day → pregabalin 300 mg/day
- Gabapentin 3600 mg/day → pregabalin 600 mg/day
Both scenarios were quick and seamless, so the authors concluded that either technique could be an effective method to switch patients between the medications.
Final thoughts
Though pregabalin and gabapentin have somewhat similar pharmacokinetic and pharmacodynamic profiles, there are clearly significant differences. Overall, pregabalin has more predictable pharmacokinetics, and it also shows a stronger binding affinity to its target receptor, increased potency, and a steeper dose-response curve in neuropathic pain that does not plateau over recommended dosing levels.
A few studies have found that pregabalin has fewer side effects and may be more efficacious for neuropathic pain than gabapentin. Several studies reviewing conversion of gabapentin to pregabalin predict that a rough ratio for conversion is about 6:1 gabapentin to pregabalin. In addition, a direct switch from gabapentin to pregabalin seems to be well tolerated, making the conversion simple.
Clinicians should note that pregabalin has various pharmacokinetic and pharmacodynamic advantages over gabapentin, and a conversion between the 2 medications is often well tolerated.
This article is the sole work of the authors and stated opinions/assertions do not reflect the opinion of employers, employee affiliates, and/or pharmaceutical companies listed.
- GABA analogue
- 4-Methylpregabalin
- Gabapentin (Neurontin, Gabarone)
- Gabapentin enacarbil (Horizant)
- Atagabalin
- Imagabalin
- Mirogabalin
- Phenibut (Noofen, Citrocard)
- Gabapentinoids
- Butyric acid (butanoic acid) – agonist of Free fatty acid receptor 2, Free fatty acid receptor 3, and Niacin receptor 1, and HDAC inhibitor
- Derivatives: butyrate (butanoate), sodium butyrate, methyl butyrate, ethyl butyrate, butyl butyrate, pentyl butyrate
- Valeric acid (pentanoic acid) – constituent of valerian; has a pleasant odor and fruity flavor and esters are used as additives
- Derivatives: valerate (pentanoate), methyl valerate, ethyl valerate, pentyl valerate
- Isovaleric acid (isopentanoic acid/3-methylbutanoic acid) – constituent of valerian; has anticonvulsant effects; PAM of the GABA receptor
- Derivatives: isovalerate (isopentanoate/3-methylbutanoate), menthyl isovalerate (validolum) – used as an anxiolytic and sedative in Russia
- Isovaleramide (isopentamide/3-methylbutanamide) – constituent of valerian; has anxiolytic and sedative effects; PAM of the GABAA receptor
- Valproic acid (2-propylpentanoic acid) – anticonvulsant/mood stabilizer; inhibitor of HDAC, SSADH, and GABA-T, blocker of VDSCs and GABA reuptake, AR/PR antagonist
- Derivatives: sodium valproate, valproate semisodium, divalproex sodium, valproate pivoxil
- Valpromide (2-propylpentanamide) – anticonvulsant; same mechanism of action as valproic acid, plus inhibitor of epoxide hydrolase
- Valnoctamide (2-ethyl-3-methylpentanamide) – anticonvulsant; similar mechanism of action to valproic acid; structural isomer of valpromide
3- or 4-Hydroxylated
- 3-Hydroxybutanal – synthetic hypnotic and sedative drug
- GHB (γ-hydroxybutyric acid) – neurotransmitter, drug of abuse; agonist of GHB receptor and GABAB receptor
- Derivatives: sodium oxybate (sodium γ-hydroxybutanoate) – used to treat narcolepsy; same mechanism of action as GHB
- Aceburic acid (γ-hydroxybutyric acid acetate) – synthetic prodrug to GHB
- GBL (γ-hydroxybutyric acid lactone) – metabolic intermediate and prodrug to GHB
- GHBAL (γ-hydroxybutyraldehyde or γ-hydroxybutanal) – metabolic intermediate and prodrug to GHB
- GHV (γ-hydroxyvaleric acid) – designer drug; analogue of GHB with similar effects
- GVL (γ-valerolactone) – designer drug; prodrug to GHV
- T-HCA/GHC (γ-hydroxycrotonic acid) – neurotransmitter; GHB receptor agonist
- GCL (γ-crotonolactone) – prodrug to T-HCA/GHC
- HOCPCA (3-hydroxycyclopent-1-enecarboxylic acid) – synthetic GHB receptor agonist
- UMB68 (γ-hydroxy-γ-methylpentanoic acid) – synthetic GHB receptor agonist
β-Substituted
- GABOB (β-hydroxy-GABA) – anticonvulsant; GABA receptor agonist
- Pregabalin (β-isobutyl-GABA) – analgesic, anticonvulsant, anxiolytic, and drug of abuse; potent inhibitor of α2δ subunit-containing VGCCs.
- Phenibut (β-phenyl-GABA) – sedative and anxiolytic from Russia; inhibitor of α2δ subunit-containing VGCCs and, to a lesser extent, GABAB receptor agonist.
- Baclofen (β-(4-chlorophenyl)-GABA) – antispasmodic drug; potent GABAB receptor agonist, weak inhibitor of α2δ subunit-containing VGCCs
- Tolibut (β-(4-methylphenyl)-GABA) – analgesic, tranquilizing, and neuroprotective drug
- Phaclofen (phosphonobaclofen) – GABAB receptor antagonist
- Saclofen (sulfonobaclofen) – GABAB receptor antagonist
Aromatized
- Arecaidine – constituent of areca nuts; GABA reuptake inhibitor
- Gabaculine – neurotoxin; GABA-T inhibitor and GABA reuptake inhibitor
- Gabapentin – anticonvulsant; inhibitor of α2δ subunit-containing VGCCs
- Gabapentin enacarbil – used for the treatment of restless legs syndrome and postherpetic neuralgia; same mechanism of action as gabapentin
- Gaboxadol – GABAA receptor agonist
- Guvacine – constituent of areca nuts; GABA reuptake inhibitor
- Isoguvacine – GABAA receptor agonist
- Isonipecotic acid – GABAA receptor partial agonist
- Muscimol – constituent of Amanita muscaria mushrooms; GABAA receptor agonist
- Nipecotic acid – used in scientific research; GABA reuptake inhibitor
GABA prodrugs
- L-Glutamine – endogenous precursor of GABA and glutamate
- N-Isonicotinoyl-GABA – structural isomer of picamilon
- Picamilon (N-nicotinoyl-GABA) – dietary supplement and prescription drug in Russia
- Progabide (complex structure) – anticonvulsant
- Tolgabide (complex structure) – anticonvulsant
Others/miscellaneous
- 1,4-Butanediol – metabolic intermediate and prodrug to GHB
- 3-Methyl-GABA – GABA-T activator
- AABA/homoalanine (α-aminobutyric acid) – used by nonribosomal peptide synthetases
- BABA (β-aminobutyric acid) – known for its ability to induce plant disease resistance
- DAVA (δ-aminopentanoic acid) – GABA receptor agonist
- Gabamide (γ-aminobutanamide) – GABA receptor agonist
- Gabazine (SR-95531) – antagonist of the GABAA and GHB receptors
- GAVA (γ-aminopentanoic acid) – GABA reuptake inhibitor
- Glutamic acid (glutamate) – neurotransmitter
- Homotaurine (tramiprosate) – GABAA receptor agonist, GABAB receptor antagonist
- Hopantenic acid (N-pantoyl-GABA) – central nervous system depressant used in Russia
- Isovaline – peripherally selective agonist of the GABAB receptor
- Lesogaberan (AZD-3355) – agonist of the GABAB receptor
- N-Anisoyl-GABA – major active metabolite of the nootropic aniracetam
- NCS-382 – antagonist of the GHB receptor
- Piracetam and other racetams – nootropics
- Pivagabine (N-pivaloyl-GABA) – antidepressant/anxiolytic drug; CRF inhibitor
- Vigabatrin (y-vinyl-GABA) – anticonvulsant; GABA-T inhibitor
Lyrica & Neurotin Face UK Restriction..Here Is Answer
Two drugs often recommended as safer alternatives to opioid pain medication could face new restrictions in the UK because of increasing reports they are being abused.
British health officials say the prescription drugs pregabalin and gabapentin, which are sold by Pfizer under the brand names Lyrica and Neurontin, are being used by drug abusers to get high, resulting in dozens of overdose deaths.
Since 2012, at least 380 deaths involving pregabalin and 260 involving gabapentin have been reported in the UK.
The prescribing of pregabalin and gabapentin in the UK has soared by 350% and 150%, respectively, in the last five years. Both medications are anti-seizure drugs widely prescribed to treat epilepsy, neuropathy, fibromyalgia and anxiety.
The UK Advisory Council on the Misuse of Drugs (ACMD) is recommending that gabapentin (Neurontin) and pregabalin (Lyrica) be reclassified as Class C controlled substances – which would mean prescriptions would only be valid for one month and there can be no refills.
“Both pregabalin and gabapentin are increasingly being reported as possessing a potential for misuse. When used in combination with other depressants, they can cause drowsiness, sedation, respiratory failure and death,” said Professor Les Iverson, ACMD chairman, in a letter to Home Office ministers.
“Pregabalin may have a higher abuse potential than gabapentin due to its rapid absorption and faster onset of action and higher potency. Pregabalin causes a ‘high’ or elevated mood in users; the side effects may include chest pain, wheezing, vision changes and less commonly, hallucinations. Gabapentin can produce feelings of relaxation, calmness and euphoria. Some users have reported that the ‘high’ from snorted gabapentin can be similar to taking a stimulant.”
The letter warns there is a risk of addiction for both drugs, as well as misuse and diversion.
“The use of gabapentin and pregabalin by the opioid abusing population either together or when opioids are unavailable reinforces the behavior patterns of this high-risk population. There is a high risk of criminal behavior stimulated by the wish to obtain gabapentin and pregabalin,” said Iverson.
Lyrica is Pfizer’s top selling drug and generates worldwide sales of over $5 billion annually. Pfizer said the recommendation to reclassify the drugs and limit their prescribing could be harmful to patients.
“We are concerned that the advice contains a number of inaccuracies and some potentially misleading information, and is contrary to the totality of the safety data available for pregabalin and gabapentin,” the company said in a statement reported on the Pulse website. “Controlling the supply of these products across the whole UK, would be a disproportionate measure that would impact on patients and their quality of life, and could also result in additional economic and operational burden on an already strained healthcare system.”
Earlier this month a study of 440 drug abusers in Ireland found that 39 tested positive for pregabalin in their urine. Only ten of them had been prescribed the drug. Other drugs detected in pregabalin positive patients were opiates, cocaine, benzodiazepine and cannabis, according to the Irish Examiner.
The study called the abuse of pregabalin a “serious emerging issue.” Recreational users of pregabalin in Belfast call the drug “Budweisers” because it induces a state similar to drunkenness.
Neurontin (gabapentin) is approved by the FDA to treat epilepsy and neuropathic pain, but is widely prescribed “off-label” for a variety of other conditions, including depression, migraines, fibromyalgia and bipolar disorder. In 1999 a Pfizer executive was so mystified by Neurontin’s growing use he called it the “snake oil of the twentieth century.”
References
- Schifano, Fabrizio (2014). “Misuse and Abuse of Pregabalin and Gabapentin: Cause for Concern?”. CNS Drugs. 28 (6): 491–6. doi:10.1007/s40263-014-0164-4. PMID 24760436.
- Pregabalin – Drugs.com”. www.drugs.com. Retrieved 2016-11-07.
- Drugs.com international listings for Gabapentin Archived 16 February 2016 at the Wayback Machine. Page accessed 9 February 2016
- “Neurontin, Gralise (gabapentin) dosing, indications, interactions, adverse effects, and more”. Medscape Reference. WebMD. Archivedfrom the original on 16 December 2014. Retrieved 6 April 2014.
- “Gabapentin Pregnancy and Breastfeeding Warnings”. Archived from the original on 8 September 2017. Retrieved 13 March 2016.
- Gabapentin”. The American Society of Health-System Pharmacists. Archived from the original on 12 January 2011. Retrieved 3 April 2011.
- Patel R, Dickenson AH (April 2016). “Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain”. Pharmacology Research & Perspectives. 4 (2): e00205. doi:10.1002/prp2.205. PMC 4804325 . PMID 27069626.
- Kriel RL, Birnbaum AK, Cloyd JC, Ricker BJ, Jones Saete C, Caruso KJ (November 1997). “Failure of absorption of gabapentin after rectal administration”. Epilepsia. 38 (11): 1242–4. doi:10.1111/j.1528-1157.1997.tb01223.x. PMID 9579927.
- Sobel SV (5 November 2012). Successful Psychopharmacology: Evidence-Based Treatment Solutions for Achieving Remission. W. W. Norton. p. 124. ISBN 978-0-393-70857-8. Archived from the original on 6 January 2016.
- Reynolds DJ, Coleman J, Aronson J (10 November 2011). Oxford Handbook of Practical Drug Therapy. Oxford University Press. p. 765. ISBN 978-0-19-956285-5. Archived from the original on 6 January 2016.
- Summary of product characteristics” (PDF). European Medicines Agency. March 6, 2013. Retrieved May 6, 2013.
- “Pregabalin (Professional Patient Advice) – Drugs.com”. www.drugs.com. Retrieved 2016-11-07.
- “Pregabalin (Professional Patient Advice)”. Drugs.com.
- Hantson, P; Courtois, F; Borrey, D; Haufroid, V (2014). “Pregabalin-associated myoclonic encephalopathy without evidence of drug accumulation in a patient with acute renal failure”. Indian Journal of Nephrology. 24 (1): 48–50. doi:10.4103/0971-4065.125102. PMC 3927193 . PMID 24574633.
- https://books.google.com/books?id=Zgx13oMZaYUC&pg=PA88&[full citation needed]
- https://www.researchgate.net/publication/237837376_Pregabalin_is_a_potent_and_selective_ligand_for_alpha2delta-1_and_alpha2delta-2_calcium_channel_subunits[full citation needed]












