Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults.
Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation.
Presbycusis refers to bilateral age-related hearing loss. In literal terms, presbycusis means “old hearing” or “elder hearing.”[rx] It becomes noticeable around age 60 and progresses slowly; however, there is evidence that certain stressors can speed the rate of deterioration. The diagnosis can be confirmed with audiometry.[rx] The hallmark of presbycusis is the impaired ability to understand high-frequency components of speech (voiceless consonants, such as p, k, f, s, and ch).[rx] There is no cure; however, hearing aids that amplify sounds can be used to mitigate symptoms. Anatomically, presbycusis involves multiple components of the auditory system. It is primarily due to age-related changes in hair cells, the stria vascularis, and afferent spiral ganglion neurons.[rx]
Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you may not realize that you’ve lost some of your ability to hear.
There are many causes of age-related hearing loss. Most commonly, it arises from changes in the inner ear as we age, but it can also result from changes in the middle ear or complex changes along the nerve pathways from the ear to the brain. Certain medical conditions and medications may also play a role.
How do we hear?
Hearing depends on a series of events that change sound waves in the air into electrical signals. Your auditory nerve then carries these signals to your brain through a complex series of steps.
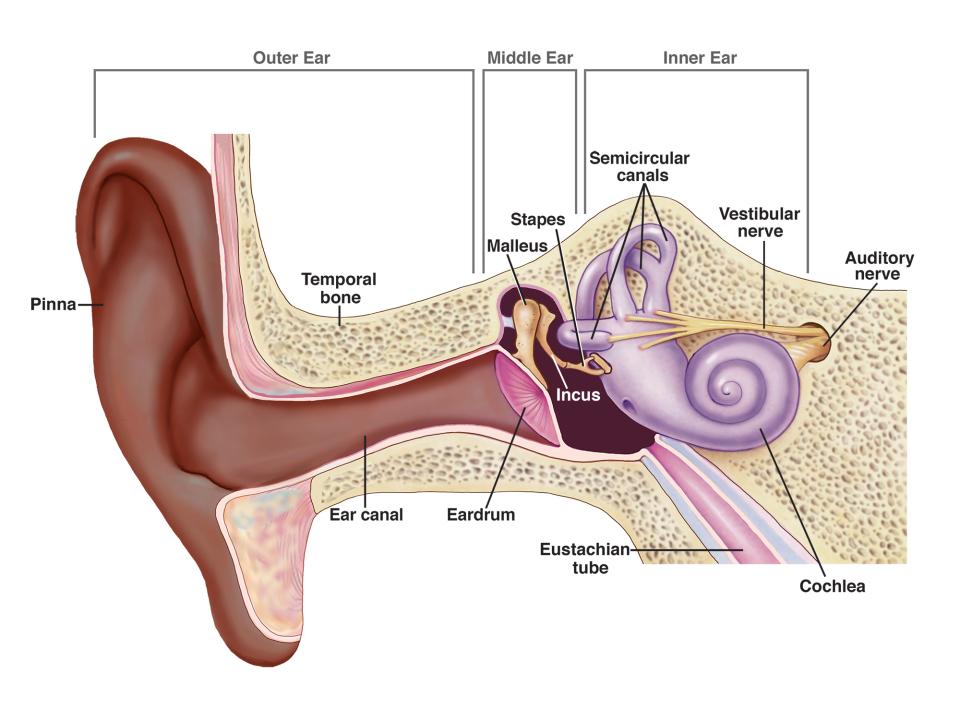
- Sound waves enter the outer ear and travel through a narrow passageway called the ear canal, which leads to the eardrum.
- The eardrum vibrates from the incoming sound waves and sends these vibrations to three tiny bones in the middle ear. These bones are called the malleus, incus, and stapes.
- The bones in the middle ear couple the sound vibrations from the air with fluid vibrations in the cochlea of the inner ear, which is shaped like a snail and filled with fluid. An elastic partition runs from the beginning to the end of the cochlea, splitting it into an upper and lower part. This partition is called the basilar membrane because it serves as the base, or ground floor, on which key hearing structures sit.
- Once the vibrations cause the fluid inside the cochlea to ripple, traveling wave forms along the basilar membrane. Hair cells-sensory cells sitting on top of the basilar membrane-ride the wave.
- As the hair cells move up and down, microscopic hair-like projections (known as stereocilia) that perch on top of the hair cells bump against an overlying structure and bend. Bending causes pore-like channels, which are at the tips of the stereocilia, to open up. When that happens, chemicals rush into the cells, creating an electrical signal.
- The auditory nerve carries this electrical signal to the brain, which turns it into a sound that we recognize and understand.
Why do we lose our hearing as we get older?
Many factors can contribute to hearing loss as you get older. It can be difficult to distinguish age-related hearing loss from hearing loss that can occur for other reasons, such as long-term exposure to noise.
Noise-induced hearing loss is caused by long-term exposure to sounds that are either too loud or last too long. This kind of noise exposure can damage the sensory hair cells in your ear that allow you to hear. Once these hair cells are damaged, they do not grow back and your ability to hear is diminished.
Conditions that are more common in older people, such as high blood pressure or diabetes, can contribute to hearing loss. Medications that are toxic to the sensory cells in your ears (for example, some chemotherapy drugs) can also cause hearing loss.
Rarely, age-related hearing loss can be caused by abnormalities of the outer ear or middle ear. Such abnormalities may include reduced function of the tympanic membrane (the eardrum) or reduced function of the three tiny bones in the middle ear that carry sound waves from the tympanic membrane to the inner ear.
Most older people who experience hearing loss have a combination of both age-related hearing loss and noise-induced hearing loss.
Causes
Presbycusis is multifactorial in origin. In addition to age-related degeneration leading to physiologic and anatomic changes, other contributing factors include genetic factors, hormones, exposure to loud noises or ototoxic agents, history of ear infection, and the presence of certain systemic diseases.[rx][rx]
Age-Related Factors
Presbycusis can be broken down further about which structures and functions are primarily affected. Some argue that there is little clinical utility in subdividing presbycusis as there is no significant change in approach or treatment, and oftentimes mixed pathology is present.[rx][rx][rx] Presently, there are thought to be six categories of presbycusis: sensory, neural, trial, mechanical, mixed, and indeterminate.[rx][rx]
-
Sensory presbycusis: loss of receptor hair cells at the basal aspect of the cochlea resulting in characteristic high-frequency hearing loss.[rx]
-
Neural presbycusis: loss of cochlear nerve fibers as well as the loss of spiral ganglion neurons.
-
Strial presbycusis: degeneration of stria vascular cells. These cells are essential for maintaining the appropriate ion composition of endolymph to generate the endocochlear potential for signal transduction.[rx] Sometimes referred to as metabolic presbycusis.
-
Mechanical presbycusis (cochlear conductive): due to physical changes of the cochlear duct. This is accompanied by a specific audiogram pattern.[rx]
-
Mixed presbycusis: characterized by pathologic changes in more than one of the above structures.
-
Indeterminate presbycusis: cases in which changes to the above structures are not significant.[rx]
Genetic Factors
Genetic factors, specifically, differences in mitochondrial DNA expression genes related to oxidative stress, have been found in patients with presbycusis when compared to controls.[rx][rx][rx]
Genetic Syndromic
In children who have hearing loss due to the syndrome, determining the underlying cause is often more important as the other clinical features can be severe.[rx] More than 400 syndromes have been identified with hearing loss as a feature; however, only a small number of these account for most cases of SNHL.[rx] Outlined below are the key features of the most common syndromes seen in children.
-
Waardenburg syndrome is the most common, with SNHL being a significant feature found in over two-thirds of patients.[rx] The other key feature is pigmentation abnormalities of the eyes, skin, and cochlea.
-
Usher syndrome is one of the most common autosomal recessive causes of syndromic hearing loss. This condition is characterized by hearing loss and visual loss due to a progressive SNHL and retinitis pigmentosa.
-
Pendred syndrome classically presents with a varying degree of SNHL, vestibular dysfunction, and a thyroid goiter. Along with Usher syndrome, it is another one of the most common autosomal recessive causes of syndromic hearing loss. A specific mutation in SLC26A4 occurs in around half of the affected patients.[rx]
-
Jervell and Lange-Nielsen syndrome is also inherited with an autosomal recessive pattern. The key feature, along with SNHL, is a prolonged QT interval seen on the ECG. These patients can suffer from or have a family history of syncope, sudden death, or long QT syndrome.
-
Alport syndrome is inherited in an X-linked manner and occurs due to a defect in type IV collagen. It classically presents with glomerulonephritis, end-stage kidney disease, eye abnormalities, and a bilateral SNHL. The hearing loss is initially in high frequency, but the lower frequencies begin to get affected as it worsens. Hematuria and proteinuria are key signs as the condition progresses.
Environmental
-
Intrauterine infection (toxoplasmosis, cytomegalovirus, herpes, rubella)
-
Alcohol, smoking
-
Ototoxic drugs
-
Premature births, hypoxia, neonatal jaundice
Ototoxic Factors
There are multiple medications associated with ototoxicity, including salicylates, loop diuretics, aminoglycoside, and certain chemotherapeutic agents.[rx][rx][rx] Additionally, some work and environmental-related exposures to chemicals such as toluene, styrene, lead, carbon monoxide, mercury, and other toxins have been shown to cause ototoxicity.[rx] Minimizing exposure to these agents can help to prevent age-related hearing loss.[rx]
Noise Exposure Factors
Some long-term studies have shown that individuals who have sustained noise-induced cochlear damage in their youth go on to develop more severe presbycusis. Anatomically, noise exposure may lead to damage and subsequent loss of spiral ganglion neurons.[rx][rx]
Hormonal Factors
Glucocorticoids, sex hormones, and glutamate signaling are thought to play a role in presbycusis.[rx] Prolonged corticosterone levels and loss of nuclear factor kappa B have been associated with increased spiral ganglion neuron loss.[rx][rx] The use of progestin and combination hormone replacement therapy in postmenopausal is associated with a more frequent incidence of hearing loss.[rx]
Sensorineural hearing loss results from damage to the hair cells within the inner ear, the vestibulocochlear nerve, or the brain’s central processing centers. This differs from conductive hearing loss, which results from the inability of sound waves to reach the inner ear.
The ear consists of
-
External ear–pinna, external auditory meatus, and canal
-
Middle ear – tympanic membrane, ossicles, Eustachian tube opening, oval and round windows
-
Inner ear – cochlea and part of the auditory nerve
Each of the above components is important for the conduction of sound waves, but in SNHL, we are concerned with pathology in the inner ear that leads to hearing loss. The interface between the stapes and the oval window delivers sound transmission to the cochlea. Sound which reaches the cochlea undergoes first amplification by the outer hair cells and then electrochemical transduction by the inner hair cells. The cochlea receives an acoustic signal, and a traveling wave is generated, which traverses the basilar membrane of the cochlea stimulating outer hair cells (OHCs), which act as a biological amplifier/compressor and modify the signal. The basilar membrane of the cochlea is highly frequency-specific and tonotopically organized. The base of the basilar membrane responds to higher-frequency sounds, while the apex responds to low frequencies. [rx]The inner hair cells (IHCs) in the cochlea transduce the energy of the traveling wave to an electric action potential and synapse at the spiral ganglion to form the auditory nerve.[rx]
There are several pathophysiological mechanisms by which damage to the inner ear results in SNHL.
-
Structural abnormality of cochlear components: e.g., trauma or congenital conditions.
-
Aberrant metabolic activity: Cochlear function is determined by the transport of ions. Genetic or acquired conditions that interfere with this transport can lead to changes in the endolymph and affect hearing.
-
Vascular: Interference with the vascular supply to the cochlea can occur in conditions such as noise trauma, ototoxicity, and systemic vascular events, which will affect the function of the stria vascularis.
-
Overcrowding of the basilar membrane preventing OHCs motility and IHCs transduction capabilities: Prevalent in conditions such as diabetes and autoimmune pathology.
-
Noise trauma: With noise trauma, the vibrational shift between the tectorial and basilar membranes increases, and this shift can damage the stereocilia of the OHCs. In turn, the stiffness of the organ of Corti decreases.[rx] Aminoglycoside antibiotics, such as gentamicin, are potassium channel blockers and stop hair cells from depolarizing. They can also change the perilymph ion concentration leading to damage to the hair cell bundle causing permanent hearing loss.
According to Schuknecht’s classification in presbycusis, three major cochlear structures can independently degenerate and influence the degree of hearing loss; afferent neurons, the organ of Corti, and stria vascularis.[rx][rx]
-
Sensory – steep high-frequency hearing loss with preserved speech perception – degeneration of the organ of Corti
-
Neural – downsloping high-frequency hearing loss with a disproportionate loss of speech perception – degeneration of spiral ganglion cells
-
Serial/metabolic – a flat SNHL with preserved speech perception – degeneration of stria vascularis
-
Cochlear conductive – progressive downsloping high-frequency SNHL – increased stiffness of the basilar membrane
Symptoms
The following are the most common symptoms of age-related hearing loss:
- Speech of others sounds mumbled or slurred
- High-pitched sounds, such as “s” or “th” are hard to distinguish
- Conversations are difficult to understand, particularly when there is background noise
- Men’s voices are easier to hear than women’s
- Some sounds seem overly loud and annoying
- Tinnitus (ringing in the ears) may occur in one or both ears
The symptoms of age-related hearing loss may look like other conditions or medical problems. Always consult your healthcare provider for a diagnosis.
Diagnosis
History and Physical
It is important to take a thorough history when assessing a patient with SNHL. Essential points to obtain include age of onset, laterality of symptoms, rapidity of decline, fluctuating symptoms, and associated symptoms such as tinnitus, aural fullness, disequilibrium, and vertigo. Establishing the premorbid hearing level is crucial to direct rehabilitation and to assess if the hearing loss is new or deterioration of an existing picture. Previous ear surgery, history of noise exposure, previous head trauma, barotrauma, or ototoxic exposure to aminoglycosides are asked.
Patients who present with presbycusis will give a history of progressive decline in hearing. They are turning up the television louder than usual and asking other people to speak up. It is often family members who notice this first. There will be a history of personal noise exposure or occupational exposure in noise-induced hearing loss cases. Social history often helps guide management and provides insight into how the patient’s symptoms affect their lives and their families. Many patients with hearing loss find it incredibly isolating. Activities they previously enjoyed, such as going to the cinema, going out to eat at a restaurant, and meeting family and friends, become stressful for them, and they withdraw from them.
When seeing patients in the clinic with a new hearing loss, aside from a focused otological examination, it is essential to do a full head and neck exam, including all the cranial nerves, although it is typically normal.
Lab Test
A complete audiometric evaluation is the gold standard for evaluating hearing loss and should be performed to evaluate someone with sensorineural hearing loss. In clinical practice, tuning fork tests, a quick and easy bedside investigation, are usually performed first alongside a pure tone audiogram (PTA) and tympanometry.
Rinne and Weber Tests
This is a bedside investigation performed using a 512Hz tuning fork and is useful when a clinician is trying to distinguish between a conductive and SNHL, though there must be at least a 20 dB difference between ears or between conduction and sensorineural thresholds for these tests to detect it.[rx][rx]
The Weber test involves striking the tuning fork against your knee and then placing the tuning fork in the middle of the patient’s forehead. The patient is then asked to identify which ear they can hear the sound loudest in. In a unilateral SNHL, the patient will hear the sound loudest in their normal or “good” ear, whereas if they had a conductive hearing loss, the sound would lateralize to their deaf or “worse” ear. If the SNHL is bilateral, then the sound will not lateralize to a particular ear.[rx]
The Rinne test is performed by first striking the tuning fork and then placing the tuning fork in two positions, firstly on the patient’s mastoid process until it is no longer heard and then approximately 1cm away from the patient’s external auditory meatus. The former is testing bone conduction and the latter is air conduction. For a normal test, or Rinne positive result, the patient will report that the sound was still heard when the fork was held in front of their ear, i.e., air conduction is better than bone conduction, implying no conductive loss. If the test yields a Rinne negative result, the patient will report that the sound was not heard when the fork is held in front of their ear, i.e., bone conduction is better than air conduction, which implies a conductive hearing loss. In SNHL, the Rinne test should be positive when the affected ear is tested as there is no conduction loss.[rx]
Pure Tone Audiogram
Patients are often sent for an audiogram to evaluate their hearing in an outpatient clinic. This essentially tests both air and bone conduction pathways, and simplistically both air and bone conduction thresholds are plotted on a graph as a curve at increasing frequencies of sound up to 8000Hz. In SNHL, both air and bone conduction curves worsen, with no air-bone gap. The shape of the curve will differ depending on the underlying pathology. For example, in presbycusis, you will see a downward-sloping high-frequency loss. Conversely, in a conductive hearing loss, the air conduction curve will worsen and shift downward, while the bone conduction curve remains the same. This difference between the two curves is the air-bone gap.
Other Tests
-
Tympanometry: It is used to assess middle ear function and the tympanic membrane. This test is often used in clinical practice to evaluate if there are otitis media with effusion and eustachian tube dysfunction. The acoustic stapedial reflex can also be assessed. The lowest intensity of sound that triggers the reflex is the acoustic reflex threshold.
-
Otoacoustic emissions are sounds recorded in the external auditory meatus and reflect the proper functioning of the OHCs. When OHCs are damaged, these sounds are absent.
-
Electrophysiological tests: Auditory brainstem testing measures nervous system activity and can be affected by cerebellopontine angle tumors compressing the cochlear nerve and neural demyelination. It is also used to predict hearing thresholds in babies.
-
Speech audiometry: This test is essential in assessing the impact of hearing loss on communication.
-
Head computed tomographic scans, including thin temporal bone window and brain magnetic resonance imaging (MRI), are also carried out to evaluate for cochlear ossification, the presence of a cerebellopontine angle tumor, or active mastoiditis.
- Laboratory tests are typically not necessary. Still, there are exceptions, such as when considering an autoimmune cause of SNHL, in which tests such as erythrocyte sedimentation rate, antinuclear antibody, rheumatoid factor, and anti-microsomal antibodies are requested.
- Otoscopy – An examination of the external ear canal and tympanic membrane performed by a medical doctor, otolaryngologist, or audiologist using an otoscope, a visual instrument inserted into the ear. This also allows some inspection of the middle ear through the translucent tympanic membrane.
- Tympanometry – A test administered by a medical doctor, otolaryngologist, or audiologist of the tympanic membrane and middle ear function using a tympanometer, an air-pressure/sound wave instrument inserted into the ear canal. The result is a tympanogram showing ear canal volume, middle ear pressure, and eardrum compliance. Normal middle ear function (Type A tympanogram) with hearing loss may suggest presbycusis. Type B and Type C tympanogram indicate an abnormality inside the ear and therefore may have an additional effect on hearing.
- Audiometry – A hearing test administered by a medical doctor, otolaryngologist (ENT), or audiologist including pure tone audiometry and speech recognition may be used to determine the extent and nature of hearing loss, and distinguish presbycusis from other kinds of hearing loss. Otoacoustic emissions and evoked response testing may be used to test for audio neuropathy. The diagnosis of a sensorineural pattern hearing loss is made through audiometry, which shows a significant hearing loss without the “air-bone gap” that is characteristic of conductive hearing disturbances. In other words, air conduction is equal to bone conduction. Persons with cochlear deficits fail otoacoustic emissions testing, while persons with 8th cranial nerve (vestibulocochlear nerve) deficits fail auditory brainstem response testing.
- Magnetic resonance imaging (MRI) – As part of differential diagnosis, an MRI scan may be done to check for vascular anomalies, tumors, and structural problems like enlarged mastoids. MRI and other types of scans cannot directly detect or measure age-related hearing loss.
Treatments
Your treatment will depend on the severity of your hearing loss, so some treatments will work better for you than others. The home has several devices and aids helpou hear better when you have hearing loss. Here are the most common ones:
Styles of hearing aids
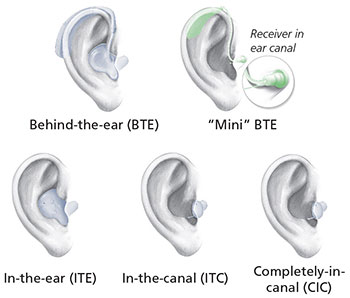
Source: NIH/NIDCD
- Hearing aids are electronic instruments you wear in or behind your ear (see figure). They make sounds louder. To find the hearing aid that works best for you, you may have to try more than one. Be sure to ask for a trial period with your hearing aid and understand the terms and conditions of the trial period. Work with your hearing aid provider until you are comfortable with putting on and removing the hearing aid, adjusting the volume level, and changing the batteries. Hearing aids are generally not covered by health insurance companies, although some do. Medicare does not cover hearing aids for adults; however, diagnostic evaluations are covered if they are ordered by a physician to assist the physician in developing a treatment plan. (Read the NIDCD fact sheet Hearing Aids for more information.)
- Cochlear implants. Cochlear (COKE-lee-ur) implants are small electronic devices surgically implanted in the inner ear that help provide a sense of sound to people who are profoundly deaf or hard of hearing. If your hearing loss is severe, your doctor may recommend a cochlear implant in one or both ears. (Read the NIDCD fact sheet Cochlear Implants for more information.)
- Bone-anchored hearing systems bypass the ear canal and middle ear and are designed to use your body’s natural ability to transfer sound through bone conduction. The sound processor picks up sound, converts it into vibrations, and then relays the vibrations through your skull bone to your inner ear.
- Assistive listening devices include telephone and cell phone amplifying devices, smartphone or tablet “apps,” and closed-circuit systems (hearing loop systems) in places of worship, theaters, and auditoriums. (Read the NIDCD fact sheet Assistive Devices for People with Hearing, Voice, Speech, or Language Disorders for more information.)
- Lip reading or speech reading is another option that helps people with hearing problems follow the conversational speech. People who use this method pay close attention to others when they talk by watching the speaker’s mouth and body movements. Special trainers can help you learn how to lip read or speech read.
Devices can be very expensive and often are not covered by the patient’s insurance.[rx][rx] While smaller hearing aids are potentially more comfortable and discrete, decreased dexterity in geriatric patients may make these devices less convenient. Importantly, the management of hearing aids does not stop once the devices are fitted. Learning to use hearing aids and adjusting to both the physical discomfort and cognitive adjustment takes significant effort and practice. A collaborative, interdisciplinary approach involving the primary care provider and audiologist is recommended for continued auditory rehabilitation.[rx] Patients often require encouragement as many find hearing aids uncomfortable, unattractive, and embarrassing.[rx] Hearing aids are indicated at certain thresholds of hearing loss. Cochlear implants can be offered to patients with severe bilateral hearing loss that is not improved with hearing aids. Specific criteria exist for patients to be considered candidates, and often include a predetermined level of impairment in word identification.
- Antioxidant therapy – the age-related hearing loss was reduced in animal models with a combination agent comprising six antioxidant agents that target four sites within the oxidative pathway: L-cysteine-glutathione mixed disulfide, ribose-cysteine, NW-nitro-L-arginine methyl ester, vitamin B12, folate, and ascorbic acid.[rx] It is thought that these supplements attenuate the decline of the cochlear structure due to prolonged oxidative stress. However, more recent studies have had conflicting results. In 2012, a study was done with CBA/J female mice. They were placed on an antioxidant-rich diet for 24 months consisting of vitamins A, C, E, L-carnitine, and α-lipoic acid. While this increased the inner ear’s antioxidant capacity, the actual hearing loss was unaffected. Therefore, in this study, antioxidants were shown not to improve presbycusis mechanisms.[rx]
- The effects of the pharmaceutical drug Tanaka were observed when treating trypanophobia in elderly women.[rx] Tanaka was found to decrease the intensity of tympanitis and improve speech and hearing in aged patients, giving rise to the idea of recommending treatment with it to elderly patients with presbycusis or normal tonal hearing.[rx]
- AM-111, an otoprotective peptide, was shown in a chinchilla study to rescue and protect against hearing loss following impulse noise trauma. AM-111 acts as a cell-permeable inhibitor of JNK-mediated apoptosis. IP injections or local injections into the membrane of the round window were given, and permanent threshold shifts (PTS) were measured three weeks after impulse noise exposure. AM-111 animals had significantly lower PTS, implicating AM-111 as a possible protective agent against JNK-mediated cochlear cell death and permanent hearing deficits after noise trauma.[rx]
- The anti-inflammatory, anti-oxidant substance Ebselen was observed to reduce hearing loss in a study done in 2007.[rx] It has been previously shown that noise trauma correlates with decreases in glutathione peroxidase (GPx) activity, which has been linked to the loss of outer hair cells. GPx1, an isoform of GPx, is predominantly expressed in stria vascularis, cochlea, spiral ligament, organ of Corti, and spiral ganglion cells. The stria vascularis displayed significant decreases in GPx1 immunoreactivity and increased swelling following noise exposure in rats. There was also significant outer hair cell loss in the cochlea within five hours of noise exposure. Administration of Ebselen before and after the noise stimulus reduced stria vascularis swelling as well as cochlear outer hair cell loss. This implicates Ebselen as a supplement for GPx1 in the outer hair cell degradation mechanism of hearing loss. This treatment is currently in active clinical trials.
- A γ-secretase inhibitor of Notch signaling was shown to induce new hair cells and partially recover the hearing loss.[rx] Auditory hair cell loss is permanent damage due to the inability of these cells to regenerate. Therefore, deafness due to this pathology is viewed as irreversible. Hair cell development is mediated by Notch signaling, which exerts lateral inhibition onto hair cells. Notch signaling in supporting hair cells leads to the prevention of differentiation in surrounding hair cells. After identifying a potent γ-secretase inhibitor selective for stimulating differentiation in inner ear stem cells, it was administered in acoustically injured mice. The animals who received the injury and treatment displayed an increased hair cell number and stimulated hearing recovery. This suggests that γ-secretase inhibition of Notch signaling can be a potential pharmacological therapy in approaching what was previously viewed as permeant deafness.
Stem cell therapy
- A fetal thymus graft, or rejuvenation of the recipient immunity by inoculation of young CD4+ T cells, also prevents presbycusis as well as up-regulation of the interleukin 1 receptor type II gene (IL1R2) in CD4+ T cells and degeneration of the spiral ganglion in Samp1 mice, a murine model of human senescence.[rx] This technology remains years or even decades away from the human application.
As extrinsic factors are thought to have a role in the progression of presbycusis, wearing earplugs or earmuffs to attenuate sounds may be helpful if the patient needs to be exposed to loud noises. A diet low in saturated fat may help slow hearing loss.[rx] Maintaining a healthy, active lifestyle is a logical form of risk reduction because hearing loss is associated with stroke, myocardial ischemia, hypertension, hyperlipidemia, and diabetes. Smoking should be discouraged, as cessation has been shown to delay age-related hearing loss.[rx]
Ongoing research is abundant regarding the genetic and metabolic components of age-related hearing loss. Due to the potential role of oxidative damage, it was thought that antioxidants might slow the progression of hearing loss. While the administration of alpha-lipoic acid has been shown to prevent age-related hearing loss in rats, an antioxidant-enriched diet in humans did not delay the progression of hearing loss. Other agents, such as coenzyme Q-10 and ginkgo Biloba, have been studied and lack sufficient evidence for use. Additionally, the use of these supplements is controversial as prolonged administration has been associated with an increase in overall mortality.[rx] There are ongoing investigations into potential gene and hormone therapies for hearing loss.
Can I prevent age-related hearing loss?
At this time, scientists don’t know how to prevent age-related hearing loss. However, you can protect yourself from noise-induced hearing loss by protecting your ears from sounds that are too loud and last too long. It’s important to be aware of potential sources of damaging noises, such as loud music, firearms, snowmobiles, lawnmowers, and leaf blowers. Avoiding loud noises, reducing the amount of time you’re exposed to loud noise, and protecting your ears with ear plugs or ear muffs are easy things you can do to protect your hearing and limit the amount of hearing you might lose as you get older.
How can I tell if I have a hearing problem?
Ask yourself the following questions. If you answer “yes” to three or more of these questions, you could have a hearing problem and may need to have your hearing checked.
Adapted from: Newman, C.W., Weinstein, B.E., Jacobson, G.P., & Hug, G.A. (1990). The Hearing Handicap Inventory for Adults [HHIA]: Psychometric adequacy and audiometric correlates. Ear Hear, 11, 430-433.
What should I do if I have trouble hearing?
Hearing problems can be serious. The most important thing you can do if you think you have a hearing problem is to seek advice from a healthcare provider. There are several types of professionals who can help you. You might want to start with your primary care physician, an otolaryngologist, an audiologist, or a hearing aid specialist. Each has a different type of training and expertise. Each can be an important part of your hearing health care.
- An otolaryngologist (oh-toe-lair-in-GAH-lush-jist) is a doctor who specializes in diagnosing and treating diseases of the ear, nose, throat, and neck. An otolaryngologist sometimes called an ENT, will try to find out why you’re having trouble hearing and offer treatment options. He or she may also refer you to another hearing professional, an audiologist.
- An audiologist (aw-dee-AH-lush-jist) has specialized training in identifying and measuring the type and degree of hearing loss. Some audiologists may be licensed to fit hearing aids.
- A hearing aid specialist is someone who is licensed by your state to conduct and evaluate basic hearing tests, offer to counsel, and fit and test hearing aids.
Can my friends and family help me?
You and your family can work together to make living with hearing loss easier. Here are some things you can do:
- Tell your friends and family about your hearing loss. The more friends and family you tell, the more people there will be to help you cope with your hearing loss.
- Ask your friends and family to face you when they talk so that you can see their faces. If you watch their faces move and see their expressions, it may help you to understand them better.
- Ask people to speak louder, but not shout. Tell them they do not have to talk slowly, just more clearly.
- Turn off the TV or the radio when you aren’t actively listening to it.
- Be aware of noise around you that can make hearing more difficult. When you go to a restaurant, for example, don’t sit near the kitchen or a band playing music. Background noise makes it hard to hear people talk.
Working together to hear better may be tough on everyone for a while. It will take time for you to get used to watching people as they talk and for people to get used to speaking louder and more clearly. Be patient and continue to work together. Hearing better is worth the effort.
References







