The gas laws are a group of physical laws modeling the behavior of gases developed from experimental observations from the 17th century onwards. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure (STP), their principles can still be useful in understanding and altering a significant number of physicochemical processes of the body as well as the mechanism of action of drugs (e.g., inhaled anesthetics).[rx]
This argument, which combines physics, medicine, physiology, and biology, starts from the assumption that pressure, volume, and temperature are interconnected variables. Indeed, each gas law holds one constant and observes the variation in the other two.
Types of Gas Laws
Boyle’s Law
Boyle’s law or Boyle–Mariotte law or Mariotte’s law (especially in France) takes the name of Robert Boyle (1627–1691) and is based on the research of Richard Towneley (1629-1707) and Henry Power (1623–1668). It states that at a constant temperature, the pressure is inversely proportional to volume:
-
P alpha 1/V or P·V = k, where k is a constant and is dependent on the temperature.
NB: alpha means ‘is proportional to.’
For the same gas under different conditions at the same temperature, it can also be expressed as:
-
P1·V1 = P2·V2
Charles’ Law
Charles’s law, discovered by Jacques Charles (1746-1823) in 1787 and refined by Joseph Louis Gay-Lussac (1778-1850) in 1808, states that at constant pressure, the volume is directly proportional to absolute temperature, for a fixed mass of a gas:
-
V alpha T, which can also be stated as V/T = k, where k is a constant, and similarly, V1/T1 = V2/T2
Gay-Lussac’s Law
Gay-Lussac’s Law or Third Gas Law states that for a constant volume, the pressure is directly proportional to absolute temperature:
-
P alpha T; also stated as P/T = K, where K is a constant, and similarly, P1/T1 = P2/T2
Those three laws can be mathematically combined and expressed as:
-
P1V1/T1 = P2V2/T2
In addition to the three fundamental laws, other gas laws must be considered.
Avogadro’s Law
Equal volumes of gases at the same temperature and pressure contain the same number of molecules (6.023·10^23, Avogadro’s number). In other words, the volume occupied by an ideal gas is proportional to the number of moles of gas and the molar volume of an ideal gas (the space occupied by 1 mole of the “ideal” gas) is 22.4 liters at standard temperature and pressure.
Ideal Gas Law
The ideal gas law is a combination of Boyle’s law, Charles’s law, Gay-Lussac’s law, and Avogadro’s law:
-
P·V = n·R·T
n is the number of moles of the gas (mol), R is the ideal gas constant (8.314 J/(K·mol), or 0.820 (L·atm)/(K·mol)), T is the absolute temperature (K), P is pressure, and V is volume.
Dalton’s Law and Henry’s Law
Dalton’s law of partial pressures states that, for a mixture of non-reacting gases, the sum of the partial pressure of each gas is equal to the total pressure exerted by the mixture, at constant temperature and volume:
-
Ptotal= P1 + P2 + … Pz, or Ptotal= (n1·R·T1/V1) + (n2·R·T2/V2) + … (nz·R·Tz/Vz)
Henry’s law states that for a constant temperature, the amount of dissolved gas in a liquid is directly proportional to the partial pressure of that gas (in contact with its surface). This relationship is no longer linear once a gas mixture is used, due to stabilization and destabilization effects on solubility[rx], and deviations are found with increasingly high pressures or concentrations[rx]:
-
P = K·M, where P is the partial pressure of the gas, K is Henry’s constant of proportionality, and M is the molar concentration of the gas.
Graham’s Law
The rate of diffusion (or effusion) of a gas is inversely proportional to the square root of the mass of its particles. When a gas had particularly large particles (or is particularly dense), it will mix more slowly with other gases, and oozes more slowly from its containers.
Dalton’s Law of Partial Pressure
Dalton’s law of partial pressures states that the pressure of a mixture of gases is the sum of the pressures of the individual components.
Key Points
This empirical law was observed by John Dalton in 1801 and is related to the ideal gas laws.
Atmospheric air is a mixture of nitrogen, water, oxygen, carbon dioxide, and other minor gasses. The relative concentrations of a gasses don’t change even as the pressure and volume of the total gasses change.
Gasses flow from areas of high to low pressure, so the partial pressures of inhaled and alveolar air determine why oxygen goes into the alveoli, and why carbon dioxide leaves the alveoli.
Dalton’s law is only completely accurate for ideal gasses.
Key Terms
- Dalton’s law: The total pressure of a mixture of gases is the sum of the partial pressures of each gas in the mixture; it is only true for ideal gases.
Dalton’s law states that the total pressure exerted by the mixture of inert (non-reactive) gases is equal to the sum of the partial pressures of individual gases in a volume of air. This empirical law was observed by John Dalton in 1801 and is related to the ideal gas laws.
Dalton’s Law in Respiration
The air in the atmosphere is a mixture of many different gases, that vary in concentration. Dalton’s law states that at any given time, the percentage of each of these gasses in the air we breathe makes its contribution to total atmospheric pressure, and this contribution will depend on how much of each gas is in the air we breathe.
Dalton’s law also implies that the relative concentration of gasses (their partial pressures) does not change as the pressure and volume of the gas mixture changes so that air inhaled into the lungs will have the same relative concentration of gasses as atmospheric air. In the lungs, the relative concentration of gasses determines the rate at which each gas will diffuse across the alveolar membranes.
Mathematically, the pressure of a mixture of gases can be defined as the sum of the partial pressures of each of the gasses in the air.
Ptotal=P1+P2+P3+⋯+Pn=∑i=1nPi
DALTON’S LAW
In regards to atmospheric air, Dalton’s law becomes:
Atm=PN2+PO2+PCO2+PH2O+P(other gasses)
For the purposes of gas exchange, O2 and CO2 are mainly considered due to their metabolic importance in gas exchange. Because gasses flow from areas of high pressure to areas of low pressure, atmospheric air has higher partial pressure of oxygen than alveolar air (PO2= 159mm Hg compared to PAO2= 100mm Hg).
Similarly, atmospheric air has a much lower partial pressure for carbon dioxide compared to alveolar air (PCO2= .3mm Hg compared to PACO2= 40mm Hg). These pressure differences explain why oxygen flows into the alveoli and why carbon dioxide flows out of the alveoli through passive diffusion (just as a similar process explains alveolar and arterial gas exchange).
While inhaled air is similar to atmospheric air due to Dalton’s law, exhaled air will have relative concentrations that are in between atmospheric and alveolar air due to the passive diffusion of gasses during gas exchange.
Dalton’s law is only truly applicable in every situation to ideal gasses. Therefore most gasses will not follow it exactly, especially in conditions of extremely high pressure, or in situations where intermolecular forces act to keep the gasses together.
Henry’s Law
Henry’s law states that the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas.
Key Points
At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Gasses with a higher solubility will have more dissolved molecules than gasses with a lower solubility if they have the same partial pressure.
Henry’s law explains how gasses dissolve across the alveoli–capillary barrier.
Henry’s law predicts how gasses behave during gas exchange based on
the partial pressure gradients and solubility of oxygen and carbon
dioxide.
Key Terms
- Henry’s law: At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
- partial pressure gradient: The difference between the partial pressures (and thus concentration) of gasses between gaseous and dissolved forms.
EXAMPLES
An everyday example of Henry’s law is given by carbonated soft drinks. Before the bottle or can is opened, the gas above the drink is almost pure carbon dioxide at a pressure slightly higher than atmospheric pressure. The drink itself contains dissolved carbon dioxide. When the bottle or can is opened, some of this gas escapes, giving the characteristic hiss (or pop in the case of a sparkling wine bottle). Because the pressure above the liquid is now lower, some of the dissolved carbon dioxides comes out of the solution as bubbles. If a glass of the drink is left in the open, the concentration of carbon dioxide in the solution will come into equilibrium with the carbon dioxide in the air, and the drink will go flat.
Henry’s law states that at a constant temperature, the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. It was formulated by William Henry in 1803.

Henry’s law: Henry’s law states that when a gas is in contact with the surface of a liquid, the amount of the gas which will go into solution is proportional to the partial pressure of that gas.
The practical description for the law is that the solubility (i.e., equilibrium) of a gas in a liquid is directly proportional to the partial pressure of that gas. In addition, the partial pressure is able to predict the tendency to dissolve simply because the gasses with higher partial pressures have more molecules and will bounce into the solution they can dissolve into more often than gasses with lower partial pressures.
Henry’s law also applies to the solubility of other substances that aren’t gaseous, such as the equilibrium of organic pollutants in water being based on the relative concentration of that pollutant in the media its suspended in.
Henry’s law can be put into mathematical terms (at constant temperature):
p=kHc
Where p is the partial pressure of the solute in the gas above the solution, c is the concentration of the solute, the solubility of the substance is k, and Henry’s law constant (H), which depends on the solute, the solvent, and the
temperature.
The solubility captures the tendency of a substance to go towards equilibrium in a solution, which explains why gasses that have the same partial pressure may have different tendencies to dissolve.
Henry’s Law in Respiration
The main application of Henry’s law in respiratory physiology is to predict how gasses will dissolve in the alveoli and bloodstream during gas exchange. The amount of oxygen that dissolves into the bloodstream is directly proportional to the partial pressure of oxygen in alveolar air.
The partial pressure of oxygen is greater in alveolar air than in deoxygenated blood, so oxygen has a high tendency to dissolve into deoxygenated blood. Conversely, the opposite is true for carbon dioxide, which has a greater partial pressure in deoxygenated blood than in the alveolar air, so it will diffuse out of the solution and back into gaseous form.
Recall that the difference in partial pressures between the bloodstream and alveoli (the partial pressure gradient) are much smaller for carbon dioxide compared to oxygen. Carbon dioxide has much higher solubility in the plasma of blood than oxygen (roughly 22 times greater), so more carbon dioxide molecules are able to diffuse across the small pressure gradient of the capillary and alveoli.
Oxygen has a larger partial pressure gradient to diffuse into the bloodstream, so its lower solubility in the blood doesn’t hinder it during gas exchange. Therefore, based on the properties of Henry’s law, both the partial pressure and solubility of oxygen and carbon dioxide determine how they will behave during gas exchange.
|
Flow-Volume loop showing successful FVC maneuver. Positive values represent expiration, negative values represent inspiration. At the start of the test both flow and volume are equal to zero (representing the volume in the spirometer rather than the lung). The trace moves clockwise for expiration followed by inspiration. After the starting point the curve rapidly mounts to a peak (the peak expiratory flow). (Note the FEV1 value is arbitrary in this graph and just shown for illustrative purposes; these values must be calculated as part of the procedure).
|
|
| MeSH | D013147 |
|---|---|
| OPS-301 code | 1-712 |
 |
|
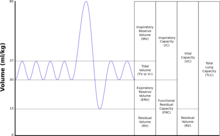
| TLC | Total lung capacity: the volume in the lungs at maximal inflation, the sum of VC and RV. |
|---|---|
| TV | Tidal volume: that volume of air moved into or out of the lungs during quiet breathing (TV indicates a subdivision of the lung; when tidal volume is precisely measured, as in gas exchange calculation, the symbol TV or VT is used.) |
| RV | Residual volume: the volume of air remaining in the lungs after a maximal exhalation |
| ERV | Expiratory reserve volume: the maximal volume of air that can be exhaled from the end-expiratory position |
| IRV | Inspiratory reserve volume: the maximal volume that can be inhaled from the end-inspiratory level |
| IC | Inspiratory capacity: the sum of IRV and TV |
| IVC | Inspiratory vital capacity: the maximum volume of air inhaled from the point of maximum expiration |
| VC | Vital capacity: the volume of air breathed out after the deepest inhalation. |
| VT | Tidal volume: that volume of air moved into or out of the lungs during quiet breathing (VT indicates a subdivision of the lung; when tidal volume is precisely measured, as in gas exchange calculation, the symbol TV or VT is used.) |
| FRC | Functional residual capacity: the volume in the lungs at the end-expiratory position |
| RV/TLC% | Residual volume expressed as a percent of TLC |
| VA | Alveolar gas volume |
| VL | The actual volume of the lung including the volume of the conducting airway. |
| FVC | Forced vital capacity: the determination of the vital capacity from a maximally forced expiratory effort |
| FEVt | Forced expiratory volume (time): a generic term indicating the volume of air exhaled under forced conditions in the first t seconds |
| FEV1 | The volume that has been exhaled at the end of the first second of forced expiration |
| FIFA | Forced expiratory flow related to some portion of the FVC curve; modifiers refer to the amount of FVC already exhaled |
| FEFmax | The maximum instantaneous flow achieved during an FVC maneuver |
| FIF | Forced inspiratory flow: (Specific measurement of the forced inspiratory curve is denoted by nomenclature analogous to that for the forced expiratory curve. For example, maximum inspiratory flow is denoted FIFmax. Unless otherwise specified, volume qualifiers indicate the volume inspired from RV at the point of measurement.) |
| PEF | Peak expiratory flow: The highest forced expiratory flow measured with a peak flow meter |
| MVV | Maximal voluntary ventilation: volume of air expired in a specified period during repetitive maximal effort |






