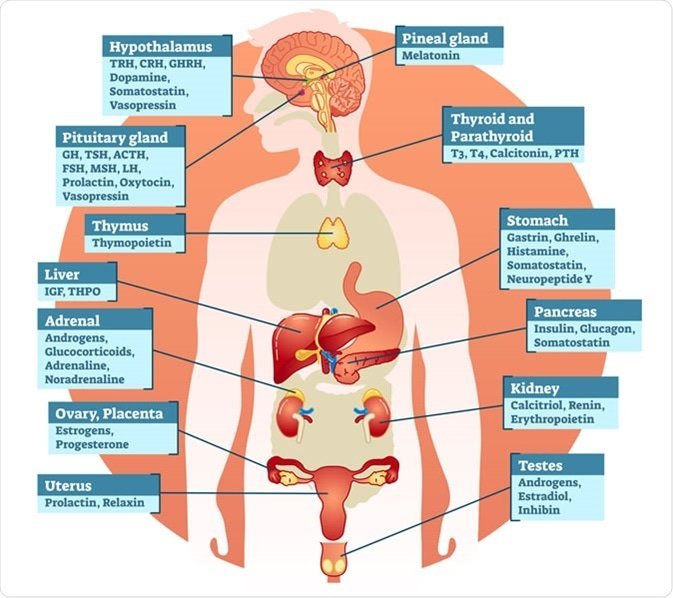
Thyroid hormone is made by the thyroid gland, a butterfly-shaped endocrine gland normally located in the lower front of the neck. Thyroid hormone is released into the blood where it is carried to all the tissues in the body. It helps the body controlling metabolism, growth, and many other body functions use energy, stay warm, and keeps the brain, heart, muscles, and other organs working as they should. The thyroid gland, anterior pituitary gland, and hypothalamus comprise a self-regulatory circuit called the hypothalamic-pituitary-thyroid axis. The main hormones produced by the thyroid gland are thyroxine or tetraiodothyronine (T4) and triiodothyronine (T3). Thyrotropin-releasing hormone (TRH) from the hypothalamus, thyroid-stimulating hormone (TSH) from the anterior pituitary gland, and T4 work in synchronous harmony to maintain proper feedback mechanism and homeostasis. Hypothyroidism, caused by an underactive thyroid gland, typically manifests as bradycardia, cold intolerance, constipation, fatigue, and weight gain. In contrast, hyperthyroidism caused by increased thyroid gland function manifests as weight loss, heat intolerance, diarrhea, fine tremor, and muscle weakness.
Iodine is an essential trace element absorbed in the small intestine. It is an integral part of T3 and T4. Sources of iodine include iodized table salt, seafood, seaweed, and vegetables. Decreased iodine intake can cause iodine deficiency and decreased thyroid hormone synthesis. Iodine deficiency can cause cretinism, goiter, myxedema coma, and hypothyroidism. [rx][rx][rx]
The thyroid gland produces three hormones:
-
Triiodothyronine, also known as T3
-
Tetraiodothyronine also called thyroxine or T4
-
Calcitonin
Strictly speaking, only T3 and T4 are proper thyroid hormones. They are made in what are known as the follicular epithelial cells of the thyroid.
Size
The thyroid gland is 2 inches (5 centimeters) wide and it weighs between 20 and 60 grams (0.7 to 2.1 ounces), according to the U.S. National Library of Medicine. The gland stretches across the front of the neck, below the voice box. Like a butterfly, it has two wings called lobes that stretch around the windpipe. The wings are connected by a small piece called the isthmus.
Cellular
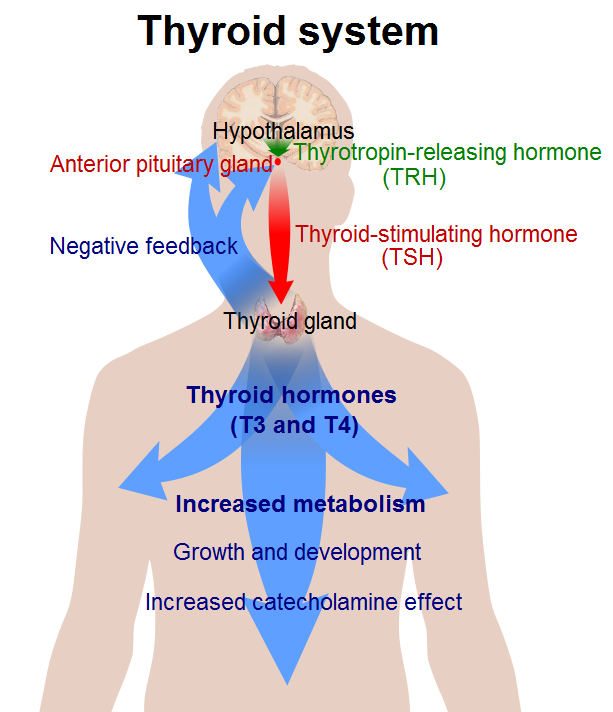
Regulation of thyroid hormone starts at the hypothalamus. The hypothalamus releases thyrotropin-releasing hormone (TRH) into the hypothalamic-hypophyseal portal system to the anterior pituitary gland. TRH stimulates thyrotropin cells in the anterior pituitary to the release of thyroid-stimulating hormone (TSH). TRH is a peptide hormone created by the cell bodies in the periventricular nucleus (PVN) of the hypothalamus. These cell bodies project their neurosecretory neurons down to the hypophyseal portal circulation, where TRH can concentrate before reaching the anterior pituitary.
TRH is a tropic hormone, meaning that it indirectly affects cells by stimulating other endocrine glands first. It binds to the TRH receptors on the anterior pituitary gland, causing a signal cascade mediated by a G-protein coupled receptor. Activation of Gq protein leads to the activation of phosphoinositide-specific phospholipase C (PLC). PLC hydrolyzes phosphatidylinositol 4,5-P(PIP) into inositol 1,4,5-triphosphate (IP) and 1,2-diacylglycerol (DAG). These second messengers mobilize intracellular calcium stores and activate protein kinase C, leading to downstream gene activation and transcription of TSH. TRH also has a nootropic effect on the pituitary gland through the hypothalamic-pituitary-prolactin axis. As a nootropic hormone, TRH directly stimulates lactotropic cells in the anterior pituitary to produce prolactin. Other substances like serotonin, gonadotropin-releasing hormone, and estrogen can also stimulate prolactin release. Prolactin can cause breast tissue growth and lactation. [rx]
TSH is released into the blood and binds to the thyroid-releasing hormone receptor (TSH-R) on the basolateral aspect of the thyroid follicular cell. The TSH-R is a Gs-protein coupled receptor, and its activation leads to the activation of adenylyl cyclase and intracellular levels of cAMP. The increased cAMP activates protein kinase A (PKA). PKA phosphorylates different proteins to modify their functions.
The five steps of thyroid synthesis are below
- Synthesis of Thyroglobulin – Thyrocytes in the thyroid follicles produce a protein called thyroglobulin (TG). TG does not contain any iodine, and it is a precursor protein stored in the lumen of follicles. It is produced in the rough endoplasmic reticulum. Golgi apparatus pack it into the vesicles, and then it enters the follicular lumen through exocytosis.
- Iodide uptake – Protein kinase A phosphorylation causes increased activity of basolateral Na+-I- symporters, driven by Na+-K+-ATPase, to bring iodide from the circulation into the thyrocytes. Iodide then diffuses from the basolateral side to the apex of the cell, where it is transported into the colloid through the Pendrin transporter.
- Iodination of thyroglobulin – Protein kinase A also phosphorylates and activates the enzyme thyroid peroxidase (TPO). TPO has three functions: oxidation, organification, and coupling reaction.
-
Oxidation – TPO uses hydrogen peroxide to oxidize iodide (I-) to iodine (I2). NADPH-oxidase, an apical enzyme, generates hydrogen peroxide for TPO.
-
Organification – TPO links tyrosine residues of thyroglobulin protein with I2. It generates monoiodotyrosine (MIT) and diiodotyrosine (DIT). MIT has a single tyrosine residue with iodine, and DIT has two tyrosine residues with iodine.
-
Coupling reaction – TPO combines iodinated tyrosine residues to make triiodothyronine (T3) and tetraiodothyronine (T4). MIT and DIT join to form T3, and two DIT molecules form T4.
-
- Storage – thyroid hormones are bound to thyroglobulin for stored in the follicular lumen.
- Release – thyroid hormones are released into the fenestrated capillary network by thyrocytes in the following steps:
-
Thyrocytes uptake iodinated thyroglobulin via endocytosis
-
Lysosome fuse with the endosome containing iodinated thyroglobulin
-
Proteolytic enzymes in the endolysosome cleave thyroglobulin into MIT, DIT, T3, and T4.
-
T3 (20%) and T4 (80%) are released into the fenestrated capillaries via MCT8 transporter. [rx]
-
Organ Systems Involved
Thyroid hormone affects virtually every organ system in the body, including the heart, CNS, autonomic nervous system, bone, GI, and metabolism. In general, when the thyroid hormone binds to its intranuclear receptor, it activates the genes for increasing metabolic rate and thermogenesis. Increasing metabolic rate involves increased oxygen and energy consumption.
- Heart – thyroid hormones have a permissive effect on catecholamines. It increases the expression of beta-receptors to increase heart rate, stroke volume, cardiac output, and contractility.
- Lungs – thyroid hormones stimulate the respiratory centers and lead to increased oxygenation because of increased perfusion.
- Skeletal muscles – thyroid hormones cause increased development of type II muscle fibers. These are fast-twitch muscle fibers capable of fast and powerful contractions.
- Metabolism – thyroid hormone increases the basal metabolic rate. It increases the gene expression of Na+/K+ ATPase in different tissues leading to increased oxygen consumption, respiration rate, and body temperature. Depending on the metabolic status, it can induce lipolysis or lipid synthesis. Thyroid hormones stimulate the metabolism of carbohydrates and anabolism of proteins. Thyroid hormones can also induce catabolism of proteins in high doses. Thyroid hormones do not change the blood glucose level, but they can cause increased glucose reabsorption, gluconeogenesis, glycogen synthesis, and glucose oxidation.
- Growth during childhood – In children, thyroid hormones act synergistically with growth hormones to stimulate bone growth. It induces chondrocytes, osteoblasts, and osteoclasts. Thyroid hormone also helps with brain maturation by axonal growth and the formation of the myelin sheath.[rx]
Functions
The physiological effects of thyroid hormones are listed below
-
Increases the basal metabolic rate
-
Depending on the metabolic status it can induce lipolysis or lipid synthesis
-
Stimulate the metabolism of carbohydrates
-
Anabolism of proteins. Thyroid hormones can also induce catabolism of proteins in high doses
-
Permissive effect on catecholamines
-
In children, thyroid hormones act synergistically with growth hormone to stimulate bone growth
-
The impact of thyroid hormone in CNS is important. During the prenatal period, it is needed for the maturation of the brain. In adults, it can affect mood. Hyperthyroidism can lead to hyperexcitability and irritability. Hypothyroidism can cause impaired memory, slowed speech, and sleepiness.
-
Thyroid hormone affects fertility, ovulation, and menstruation
- Regulate the rate at which calories are burned, affecting weight loss or weight gain.
- Can slow down or speed up the heartbeat.
- Can raise or lower body temperature.
- Influence the rate at which food moves through the digestive tract.
- Control the way muscles contract.
- Control the rate at which dying cells are replaced.
Mechanism
Thyroid hormones are lipophilic and circulate bound to the transport proteins. Only a fraction (~0.2%) of the thyroid hormone (free T4) is unbound and active. Transporter proteins include thyroxine-binding globulin (TBG), transthyretin, and albumin. TBG transports the majority (two-thirds) of the T4, and transthyretin transports thyroxine and retinol. When it reaches its target site, T3 and T4 can dissociate from their binding protein to enter cells either by diffusion or carrier-mediated transport. Receptors for T3 bind are already bound to the DNA in the nucleus before the ligand binding. T3 or T4 then bind to nuclear alpha or beta receptors in the respective tissue and cause activation of transcription factors leading to the activation of certain genes and cell-specific responses. Thyroid hormones are degraded in the liver via sulfation and glucuronidation and excreted in the bile. [rx]
Thyroid receptors are transcription factors that can bind to both T3 and T4. However, they have a much higher affinity for T3. As a result, T4 is relatively inactive. Deiodinases convert T4 to active T3 or inactive reverse T3 (rT3). There are three types of deiodinases: type I, II, and III. Type I (DIO1) and II (DIO2) are located in the liver, kidneys, muscles, and thyroid glands. Type III (DIO3) deiodinases are located in the CNS and placenta. DIO1 and DIO2 convert T4 to active form T3, and DIO3 converts T4 into inactive form rT3. [rx]
Clinical Significance
Symptoms of Hypothyroidism
Generalized decreased basal metabolic rate can present as apathy, slowed cognition, skin dryness, alopecia, increased low-density lipoproteins, and increased triglycerides. Hypothyroidism must be ruled out in psychiatry patients presenting with apathy and slowed cognition. Hypothyroidism can decrease sympathetic activity leading to decreased sweating, bradycardia, and constipation. Patients can present with myopathy and decreased cardiac output because of decreased transcription of sarcolemmal genes.
Hyperprolactinemia can be caused by hypothyroidism. Thyrotropin-releasing hormone (TRH) from the hypothalamus stimulates prolactin and TSH release. Prolactin release can suppress testosterone, LH, FSH, and GnRH release. Prolactin can also cause breast tissue growth.
Patients with hypothyroidism may present with myxedema caused by decreased clearance of complex glycosaminoglycans and hyaluronic acids from the reticular layer of the dermis. Initially, the nonpitting edema is pretibial. As the state of hypothyroidism continues, patients can develop generalized edema.
- Feeling cold when other people do not
- Constipation
- Muscle weakness
- Weight gain, even though you are not eating more food
- Joint or muscle pain
- Feeling sad or depressed
- Feeling very tired
- Pale, dry skin
- Dry, thinning hair
- Slow heart rate
- Less sweating than usual
- A puffy face
- A hoarse voice
- More than usual menstrual bleeding
Symptoms related to decreased metabolic rate:
-
Bradycardia
-
Fatigue
-
Cold intolerance
-
Weight gain
-
Poor appetite
-
Hair loss
-
Cold and dry skin
-
Constipation
-
Myopathy, stiffness, cramps, entrapment syndromes
-
Delayed deep tendon reflex relaxation
Symptoms from generalized myxedema:
-
Myxedematous heart disease
-
Puffy appearance with doughy skin texture
-
Hoarse voice with difficulty articulate words
-
Pretibial and periorbital edema
Symptoms of hyperprolactinemia:
-
Amenorrhea or menorrhagia
-
Galactorrhea
-
Erectile dysfunction, infertility in men
-
Decreased libido
Other symptoms:
-
Depression
-
Impaired concentration and memory
-
Goiter
-
Hypertension
Congenital hypothyroidism:
-
Umbilical hernia
-
Hypotonia
-
Prolonged neonatal jaundice
-
Poor feeding, absence of thirst (adipsia)
-
Decreased activity
-
Pot-belly, puffy-face, protuberant tongue
-
Poor brain development
Symptoms of Hyperthyroidism
Generalized hypermetabolism from hyperthyroidism causes increased Na+/K+-ATPase to promote thermogenesis. There is increased catecholamine secretion and, beta-adrenergic receptors are also upregulated in various tissues. As a result of the hyperadrenergic state, peripheral vascular resistance is decreased. In the heart, hyperthyroidism causes a decreased amount of phospholamban, a protein that normally decreases the affinity of calcium-ATPase for calcium in the sarcoplasmic reticulum. As a result of decreased phospholamban, there is increased Ca+ movement between the sarcoplasmic reticulum and cytosol, leading to increased contractility. Increased beta-receptors on the heart also leads to increased cardiac output.
General
-
Heat intolerance
-
Weight loss
-
Increased appetite
-
Increased sweating from cutaneous blood flow increase
-
Weakness
-
Fatigue
-
Onycholysis (separation of nails from nail beds)
-
Pretibial myxedema
- Weight loss, even if you eat the same or more food (most but not all people lose weight)
- Eating more than usual
- Rapid or irregular heartbeat or pounding of your heart
- Feeling nervous or anxious
- Feeling irritable
- Trouble sleeping
- Trembling in your hands and fingers
- Increased sweating
- Feeling hot when other people do not
- Muscle weakness
- Diarrhea or more bowel movements than normal
- Fewer and lighter menstrual periods than normal
- Changes in your eyes can include bulging of the eyes, redness, or irritation
Eyes
-
Lid lag (when looking down, sclera visible above cornea)
-
Lid retraction (when looking straight, sclera visible above the cornea)
-
Graves ophthalmopathy
Goiter
-
Diffuse, smooth, non-tender goiter
-
The audible bruit can be heard at the superior poles
Cardiovascular
-
Tachycardia (can be masked by patients taking beta-blockers)
-
Palpitations
-
An irregular pulse from atrial fibrillation
-
Hypertension
-
Widened pulse pressure because systolic pressure increases and diastolic pressure decreases
-
Heart failure (elderly patients)
-
Chest pain
-
Abnormal heart rhythms
Musculoskeletal
-
Fine tremors of the outstretched fingers. Face, tongue, and head can also be involved. Tremors respond well to treatment with beta-blockers.
-
Myopathy affecting proximal muscles. Serum creatine kinase levels can be normal
-
Osteoporosis caused by the direct effects of T3. Elderly patients can present with fractures.
Neuropsychiatric system
-
Restlessness
-
Anxiety
-
Depression
-
Emotional instability
-
Insomnia
-
Tremoulousness
-
Hyperreflexia
Conditions associated with hypothyroidism
-
Iodine deficiency [rx]
-
Cretinism [rx]
-
Wolff-Chaikoff effect [rx]
-
Subacute thyroiditis [rx]
-
Postpartum thyroiditis [rx]
-
Riedel thyroiditis [rx]
-
Hashimoto thyroiditis [rx]
-
Drug-induced [rx]
Conditions associated with hyperthyroidism
-
Graves disease [rx]
-
Iodine excess [rx]
-
Struma ovarii [rx]
-
Thyrotropic pituitary adenoma [rx]
-
Job-Basedow phenomenon [rx]
-
Drug-induced: amiodarone, lithium [rx]
-
Thyrotoxicosis and thyroid storm [rx]
-
Toxic multinodular goiter [rx]
-
Thyroid adenoma [rx]
Antithyroid drugs that work in the thyroid gland [rx]
-
Perchlorate – inhibits Na+/I- symporter – blocks iodide uptake
-
Thionamides – inhibits TPO – block thyroid hormone synthesis
-
Iodide > 5mg – inhibits Na+/I- symporter and TPO – blocks iodide uptake and thyroid hormone synthesis
-
Lithium – inhibits thyroid hormone release (off-label use for thyroid storm)
Antithyroid drugs work in peripheral tissue – all these drugs inhibit the deiodinase enzymes. Deiodinase enzymes normally convert T4 into the active form T3. These drugs inhibit the conversion of T4 to T3 and reduce its activity.
-
Propylthiouracil (ethionamide)
-
Dexamethasone
-
Amiodarone
-
Propranolol
Thyroid Conditions
- Goiter – A general term for thyroid swelling. Goiters can be harmless or can represent iodine deficiency or a condition associated with thyroid inflammation called Hashimoto’s thyroiditis.
- Thyroiditis – Inflammation of the thyroid, usually from a viral infection or autoimmune condition. Thyroiditis can be painful or have no symptoms at all. This disorder can be either painful or not felt at all. In thyroiditis, the thyroid releases hormones that were stored there. This can last for a few weeks or months.
- Hyperthyroidism – Excessive thyroid hormone production. Hyperthyroidism is most often caused by Graves disease or an overactive thyroid nodule.
- Hypothyroidism – low production of thyroid hormone. Thyroid damage caused by autoimmune disease is the most common cause of hypothyroidism.
- Graves disease – An autoimmune condition in which the thyroid is overstimulated, causing hyperthyroidism.
- Thyroid cancer – An uncommon form of cancer, thyroid cancer is usually curable. Surgery, radiation, and hormone treatments may be used to treat thyroid cancer.
- Thyroid nodule – A small abnormal mass or lump in the thyroid gland. Thyroid nodules are extremely common. Few are cancerous. They may secrete excess hormones, causing hyperthyroidism, or cause no problems.
- Thyroid storm – A rare form of hyperthyroidism in which extremely high thyroid hormone levels cause severe illness.
- Nodules – Hyperthyroidism can be caused by nodules that are overactive within the thyroid. A single nodule is called a toxic autonomously functioning thyroid nodule, while a gland with several nodules is called a toxic multi-nodular goiter.
- Excessive iodine – When you have too much iodine (the mineral that is used to make thyroid hormones) in your body, the thyroid makes more thyroid hormones than it needs. Excessive iodine can be found in some medications (amiodarone, a heart medication) and cough syrups.
- Hashimoto’s thyroiditis – A painless disease, Hashimoto’s thyroiditis is an autoimmune condition where the body’s cells attack and damage the thyroid. This is an inherited condition.
- Postpartum thyroiditis – This condition occurs in 5% to 9% of women after childbirth. It’s usually a temporary condition.
- Iodine deficiency – Iodine is used by the thyroid to produce hormones. An iodine deficiency is an issue that affects several million people around the world.
- A non-functioning thyroid gland – Sometimes, the thyroid gland doesn’t work correctly from birth. This affects about 1 in 4,000 newborns. If left untreated, the child could have both physical and mental issues in the future. All newborns are given a screening blood test in the hospital to check their thyroid function.
Thyroid Tests
- Anti-TPO antibodies – In autoimmune thyroid disease, proteins mistakenly attack the thyroid peroxidase enzyme, which is used by the thyroid to make thyroid hormones.
- Thyroid ultrasound – A probe is placed on the skin of the neck, and reflected sound waves can detect abnormal areas of thyroid tissue.
- Thyroid scan – A small amount of radioactive iodine is given by mouth to get images of the thyroid gland. Radioactive iodine is concentrated within the thyroid gland.
- Thyroid biopsy – A small amount of thyroid tissue is removed, usually to look for thyroid cancer. A thyroid biopsy is typically done with a needle.
- Thyroid-stimulating hormone (TSH) – secreted by the brain, TSH regulates thyroid hormone release. A blood test with high TSH indicates low levels of thyroid hormone (hypothyroidism), and low TSH suggests hyperthyroidism. It is produced in the pituitary gland and regulates the balance of thyroid hormones — including T4 and T3 — in the bloodstream. This is usually the first test your provider will do to check for thyroid hormone imbalance. Most of the time, thyroid hormone deficiency (hypothyroidism) is associated with an elevated TSH level, while thyroid hormone excess (hyperthyroidism) is associated with a low TSH level. If TSH is abnormal, measurement of thyroid hormones directly, including thyroxine (T4) and triiodothyronine (T3) may be done to further evaluate the problem. Normal TSH range for an adult: 0.40 – 4.50 mIU/mL (milli-international units per liter of blood).
- T4 Thyroxine tests – for hypothyroidism and hyperthyroidism, and used to monitor treatment of thyroid disorders. Low T4 is seen with hypothyroidism, whereas high T4 levels may indicate hyperthyroidism. Normal T4 range for an adult: 5.0 – 11.0 ug/dL (micrograms per deciliter of blood).
- FT4 Free T4 or free thyroxine is a method of measuring T4 that eliminates the effect of proteins that naturally bind T4 and may prevent accurate measurement. Normal FT4 range for an adult: 0.9 – 1.7 ng/dL (nanograms per deciliter of blood)
- T3 Triiodothyronine tests– help diagnose hyperthyroidism or to show the severity of hyperthyroidism. Low T3 levels can be observed in hypothyroidism, but more often this test is useful in the diagnosis and management of hyperthyroidism, where T3 levels are elevated. Normal T3 range: 100 – 200 ng/dL (nanograms per deciliter of blood).
- FT3 Free T3 or free triiodothyronine – is a method of measuring T3 that eliminates the effect of proteins that naturally bind T3 and may prevent accurate measurement. Normal FT3 range: 2.3 – 4.1 pg/mL (picograms per milliliter of blood)
- T3 and T4 (thyroxine) – The primary forms of thyroid hormone, checked with a blood test.
- Thyroglobulins – A substance secreted by the thyroid that can be used as a marker of thyroid cancer. It is often measured during follow-up in patients with thyroid cancer. High levels indicate recurrence of the cancer.
- Other imaging tests – If thyroid cancer has spread (metastasized), tests such as CT scans, MRI scans, or PET scans can help identify the extent of spread.
Related Testing
- Hypothalamus releases thyrotropin-releasing hormone (TRH) – which stimulates the secretion of TSH in the pituitary gland. Increased free T4 and T3 inhibit the release of TRH and TSH through a negative feedback loop. As a result, T3 and T4 secretion, and iodine uptake are reduced. Other hormones, such as somatostatin, glucocorticoids, and dopamine, also inhibit TSH production. Cold, stress, and exercise increase TRH release.
- TSH and free thyroxine (free T4) test – These determine whether the abnormality arises centrally from the thyroid gland (primary), peripherally from the pituitary (secondary), or hypothalamus (tertiary). In primary hypothyroidism is suspected, the thyroid gland is not releasing enough thyroid hormones. Therefore, TSH levels will be appropriately elevated, while free T4 levels will be lower. In primary hyperthyroidism, free T4 levels abnormally increased, and TSH levels will be appropriately decreased. Other lab tests such as TSH receptor antibodies or antibodies to thyroid peroxidase can help aid in the diagnosis of Graves disease or Hashimoto thyroiditis, respectively.[rx]
- Thyroid-binding globulin production – is increased because of estrogen and beta-human chorionic gonadotropin (beta-HCG). More free T4 will be bound to TGB, leading to increased production of T4. TSH levels and free T4 levels will normalize, and total T4 will increase. Therefore, laboratory values will show normal TSH, normal free T4, and elevated total T4. [rx]
Additional blood tests might include
- Thyroid antibodies – These tests help identify different types of autoimmune thyroid conditions. Common thyroid antibody tests include microsomal antibodies (also known as thyroid peroxidase antibodies or TPO antibodies), thyroglobulin antibodies (also known as TG antibodies), and thyroid receptor antibodies (includes thyroid-stimulating immunoglobulins [TSI] and thyroid blocking immunoglobulins [TBI]).
- Calcitonin – This test is used to diagnose C-cell hyperplasia and medullary thyroid cancer, both of which are rare thyroid disorders.
- Thyroglobulin – This test is used to diagnose thyroiditis (thyroid inflammation) and to monitor the treatment of thyroid cancer.
Thyroid Treatments
- Thyroid surgery (thyroidectomy) – A surgeon removes all or part of the thyroid in an operation. Thyroidectomy is performed for thyroid cancer, goiter, or hyperthyroidism.
- Antithyroid medications – Drugs can slow down the overproduction of thyroid hormone in hyperthyroidism. Two common antithyroid medicines are methimazole and propylthiouracil.
- Radioactive iodine – Iodine with radioactivity that can be used in low doses to test the thyroid gland or destroy an overactive gland. Large doses can be used to destroy cancerous tissue.
- External radiation – A beam of radiation is directed at the thyroid, on multiple appointments. The high-energy rays help kill thyroid cancer cells.
- Thyroid hormone pills – Daily treatment that replaces the amount of thyroid hormone you can no longer make. Thyroid hormone pills treat hypothyroidism and are also used to help prevent thyroid cancer from coming back after treatment.
- Recombinant human TSH – Injecting this thyroid-stimulating agent can make thyroid cancer show up more clearly on imaging tests
- Anti-thyroid drugs (methimazole and propylthiouracil) – Are medications that stop your thyroid from making hormones.
- Levothyroxine – is the standard of care in thyroid hormone replacement therapy and treatment of hypothyroidism. Levothyroxine (also called LT4) is equivalent to the T4 form of naturally occurring thyroid hormone and is available in generic and brand name forms. To optimize absorption of your thyroid medication, it should be taken with water at a regular time each day. Multiple medications and supplements decrease absorption of thyroid hormone and should be taken 3-4 hours apart, including calcium and iron supplements, proton pump inhibitors, soy, and multivitamins with minerals. Because of the way levothyroxine is metabolized by the body, your doctor may ask you to take an extra pill or skip a pill on some days of the week. This helps us to fine-tune your medication dose for your body and should be guided by an endocrinologist.
- Liothyronine – is a replacement T3 (triiodothyronine) thyroid hormone. This medication has a short half-life and is taken twice per day or in combination with levothyroxine. Liothyronine alone is not used for the treatment of hypothyroidism long term.
- Other formulations of thyroid hormone replacement include natural or desiccated thyroid hormone extracts from animal sources. Natural or desiccated thyroid extract preparations have greater variability in the dose of thyroid hormone between batches and imbalanced ratios if T4 vs T3. Natural or animal sources of thyroid hormone typically contain 75% T4 and 25% T3, compared to the normal human balance of 95% T4 and 5% T3. Treatment with a correct balance of T4 and T3 is important to replicate normal thyroid function and prevent adverse effects of excess T3, including osteoporosis, heart problems, and mood and sleep disturbance. An endocrinologist can evaluate symptoms and thyroid tests to help balance thyroid hormone medications.
| A LISTING OF THE FDA-APPROVED MEDICINES | ||
| PRODUCT |
FDA RATING
|
MANUFACTURER
|
| Unithroid® |
AB
|
(Stevens)*+ |
| L-Thyroxin |
AB
|
(Mylan) *# |
| Levo-T® |
BX
|
(Alara) |
| Levoxyl® |
BX
|
(Jones)* |
| Novothyrox® |
BX
|
(GenPharm) |
| Synthroid® |
BX
|
(Abbott)* |
| Levothroid® |
BX
|
(Forest/ Lloyd)* |
| Levolet® |
BX
|
(Vintage) |
| Tirosint® |
None
|
(IBSA) |
| LEGEND: | ||
| AB = interchangeable | ||
| BX = not interchangeable | ||
| * = currently available | ||
| + = This is BX rated vs the other name brand LT4s | ||
| # = This is AB rated only to Unithroid and is considered the only “generic”. | ||
Physiologic Effects of Thyroid Hormones
It is likely that all cells in the body are targets for thyroid hormones – While not strictly necessary for life, thyroid hormones have profound effects on many “big time” physiologic processes, such as development, growth, and metabolism, and deficiency in thyroid hormones is not compatible with normal health. Additionally, many of the effects of thyroid hormone have been delineated by the study of deficiency and excess states, as discussed briefly below.
Metabolism – Thyroid hormones stimulate diverse metabolic activities in most tissues, leading to an increase in basal metabolic rate. One consequence of this activity is to increase body heat production, which seems to result, at least in part, from increased oxygen consumption and rates of ATP hydrolysis. By way of analogy, the action of thyroid hormones is akin to blowing on a smoldering fire. A few examples of specific metabolic effects of thyroid hormones include:
- Lipid metabolism – Increased thyroid hormone levels stimulate fat mobilization, leading to increased concentrations of fatty acids in plasma. They also enhance the oxidation of fatty acids in many tissues. Finally, plasma concentrations of cholesterol and triglycerides are inversely correlated with thyroid hormone levels – one diagnostic indiction of hypothyroidism is increased blood cholesterol concentration.
- Carbohydrate metabolism – Thyroid hormones stimulate almost all aspects of carbohydrate metabolism, including enhancement of insulin-dependent entry of glucose into cells and increased gluconeogenesis and glycogenolysis to generate free glucose.
Growth – Thyroid hormones are clearly necessary for normal growth in children and young animals, as evidenced by the growth-retardation observed in thyroid deficiency. Not surprisingly, the growth-promoting effect of thyroid hormones is intimately intertwined with that of growth hormone, a clear indication that complex physiologic processes like growth depend upon multiple endocrine controls.
Development – A classical experiment in endocrinology was the demonstration that tadpoles deprived of thyroid hormone failed to undergo metamorphosis into frogs. Of critical importance in mammals is the fact that normal levels of thyroid hormone are essential to the development of the fetal and neonatal brain.
Other Effects – As mentioned above, there do not seem to be organs and tissues that are not affected by thyroid hormones. A few additional, well-documented effects of thyroid hormones include:
- Cardiovascular system – Thyroid hormones increase heart rate, cardiac contractility, and cardiac output. They also promote vasodilation, which leads to enhanced blood flow to many organs.
- Central nervous system – Both decreased and increased concentrations of thyroid hormones lead to alterations in mental state. Too little thyroid hormone and the individual tends to feel mentally sluggish, while too much induces anxiety and nervousness.
- Reproductive system – Normal reproductive behavior and physiology is dependent on having essentially normal levels of thyroid hormone. Hypothyroidism in particular is commonly associated with infertility.
How long does it take to recover from thyroid surgery (thyroidectomy)?
It will take your body a few weeks to recover after your thyroid is surgically removed (thyroidectomy). During this time you should avoid a few things, including:
- Submerging your incision underwater.
- Lifting an object that’s heavier than 15 pounds.
- Doing more than light exercise.
This generally lasts for about two weeks. After that, you can return to your normal activities.
How long after my thyroid is removed will my tiredness go away?
Typically, you will be given medication to help with your symptoms right after surgery. Your body actually has thyroid hormone still circulating throughout it, even after the thyroid has been removed. The hormones can still be in your body for two to three weeks. Medication will reintroduce new hormones into your body after the thyroid has been removed. If you are still feeling tired after surgery, remember that this can be a normal part of recovering from any type of surgery. It takes time for your body to heal. Talk to your healthcare provider if you are still experiencing fatigue and other symptoms of thyroid disease after surgery.
If part of my thyroid is surgically removed, will the other part be able to make enough thyroid hormones to keep me off of medication?
Sometimes, your surgeon may be able to remove part of your thyroid and leave the other part so that it can continue to create and release thyroid hormones. This is most likely in situations where you have a nodule that’s causing your thyroid problem. About 75% of people who have only one side of the thyroid removed are able to make enough thyroid hormone after surgery without hormone replacement therapy.
Can I check my thyroid at home?
You can do a quick and easy self-exam of your thyroid at home. The only tools you need to do this self-exam are a mirror and a glass of water.
To do the thyroid self-exam, follow these steps:
- Start by identifying where your thyroid is located. Generally, you’ll find the thyroid on the front of your neck, between your collar bone and Adam’s apple. In men, Adam’s apple is much easier to see. For women, it’s usually easiest to look from the collar bone up.
- Tip your head back while looking in a mirror. Look at your neck and try to hone in on the space you will be looking for once you start the exam.
- Once you’re ready, take a drink of water while your head is tilted back. Watch your thyroid as you swallow. During this test, you’re looking for lumps or bumps. You may be able to see them when you swallow the water.
Repeat this test a few times to get a good look at your thyroid. If you see any lumps or bumps, reach out to your healthcare provider.
Should I exercise if I have thyroid disease?
Regular exercise is an important part of a healthy lifestyle. You do not need to change your exercise routine if you have thyroid disease. Exercise does not drain your body’s thyroid hormones and it shouldn’t hurt you to exercise. It is important to talk to your healthcare provider before you start a new exercise routine to make sure that it’s a good fit for you.
Can I live a normal life with thyroid disease?
Thyroid disease is often a life-long medical condition that you will need to manage constantly. This often involves a daily medication. Your healthcare provider will monitor your treatments and make adjustments over time. However, you can usually live a normal life with a thyroid disease. It may take some time to find the right treatment option for you and control your hormone levels, but then people with these types of conditions can usually live life without many restrictions.
References




 Shop From Rxharun..
About Us...
Editorial Board Members..
Developers Team...
Team Rxharun.
Shop From Rxharun..
About Us...
Editorial Board Members..
Developers Team...
Team Rxharun.